857297
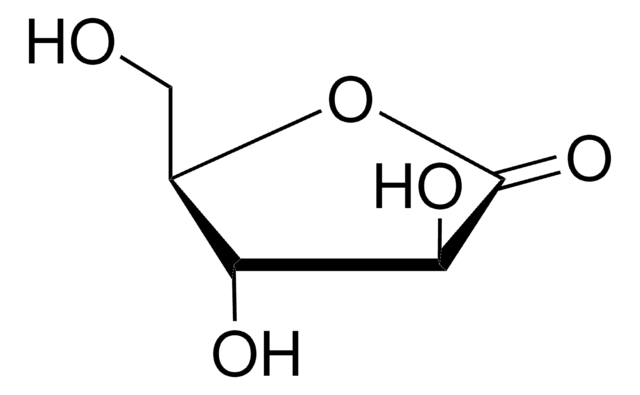
D-(+)-Ribonic γ-lactone
97%
Synonym(s):
D(+)-Ribono-1,4-lactone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H8O5
CAS Number:
Molecular Weight:
148.11
Beilstein:
82057
EC Number:
MDL number:
UNSPSC Code:
12352202
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
crystals
optical activity
[α]24/D +18°, c = 1 in H2O
mp
85-87 °C (lit.)
SMILES string
OC[C@H]1OC(=O)[C@H](O)[C@@H]1O
InChI
1S/C5H8O5/c6-1-2-3(7)4(8)5(9)10-2/h2-4,6-8H,1H2/t2-,3-,4-/m1/s1
InChI key
CUOKHACJLGPRHD-BXXZVTAOSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Important building block for chiral acyclics, cyclopentenones, and oxabicyclic systems. Also employed in studies on nonlinear optical materials.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Aldrichimica Acta, 22, 49-49 (1989)
Chemistry of Materials, 5, 802-802 (1993)
Jan-Moritz Sutter et al.
FEMS microbiology letters, 364(13) (2017-09-01)
Haloferax volcanii degrades the pentoses D-xylose and L-arabinose via an oxidative pathway to α-ketoglutarate as an intermediate. The initial dehydrogenases of the pathway, D-xylose dehydrogenase (XDH) and L-arabinose dehydrogenase (L-AraDH) catalyze the NADP+ dependent D-xylose and L-arabinose oxidation. It is
P C Raveendranath et al.
Carbohydrate research, 253, 207-223 (1994-02-03)
A series of 3-C-alkyl- (and 3-C-phenyl-) 2,3-dideoxy-D-erythro-pentono-1,4-lactones, compounds which are important in the synthesis of modified nucleosides and antibiotic sugars, were synthesized from D-ribonolactone. By a route that proceeded via 5-O-protected D-ribonolactone, 5-O-protected 2,3-dideoxy-D-glycero-pent-2-enono-1,4-lactones were synthesized and reacted with R2CuLi
High resolution gas chromatographic/real-time high resolution mass spectrometric identification of organic acids in human urine.
S Lewis et al.
Analytical chemistry, 51(8), 1275-1285 (1979-07-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service