All Photos(2)
About This Item
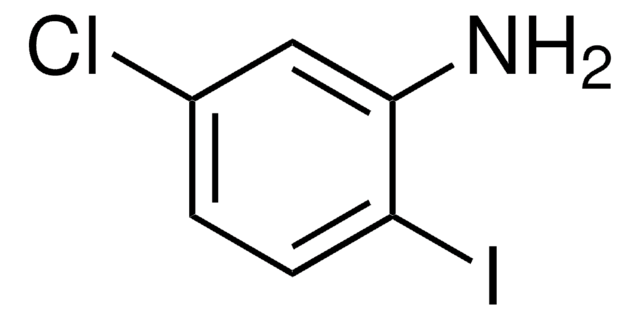
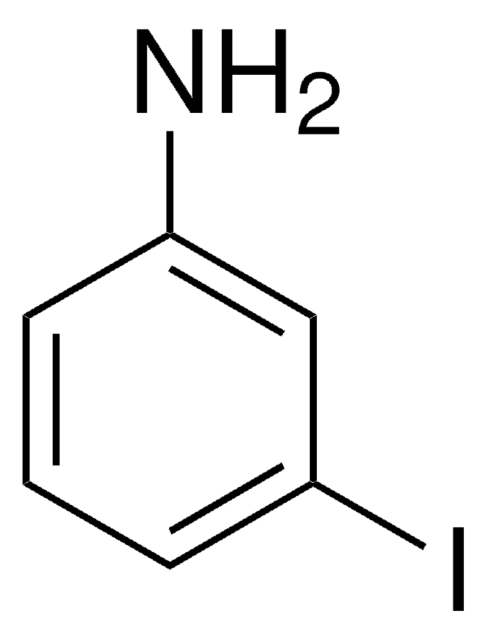
Linear Formula:
ClC6H3(I)NH2
CAS Number:
Molecular Weight:
253.47
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
39-43 °C (lit.)
functional group
chloro
iodo
SMILES string
Nc1ccc(Cl)cc1I
InChI
1S/C6H5ClIN/c7-4-1-2-6(9)5(8)3-4/h1-3H,9H2
InChI key
FLEJOBRWKBPUOX-UHFFFAOYSA-N
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
>228.2 °F - closed cup
Flash Point(C)
> 109 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Regioselective iodination of aryl amines using 1, 4-dibenzyl-1, 4-diazoniabicyclo [2.2. 2] octane dichloroiodate in solution and under solvent-free conditions.
Alikarami M, et al.
Bulletin of the Chemical Society of Ethiopia, 29(1), 157-162 (2015)
Quinazolines. VI. Synthesis of 2,4-diaminoquinazolines from anthranilonitriles.
A Rosowsky et al.
Journal of medicinal chemistry, 13(5), 882-886 (1970-09-01)
Stereoselective Synthesis of (E)-3-(Methoxycarbonyl) methylene-1, 3-dihydroindol-2-ones by Palladium-Catalyzed Oxidative Carbonylation of 2-Ethynylanilines.
Gabriele B, et al.
European Journal of Organic Chemistry, 24, 4607-4613 (2001)
Wing S Cheung et al.
The Journal of organic chemistry, 70(9), 3741-3744 (2005-04-23)
[reaction: see text] An efficient and versatile method for stereoselective synthesis of (E)-3,3-(diarylmethylene)indolinones by a palladium-catalyzed tandem Heck-carbocyclization/Suzuki-coupling sequence is presented. Factors influencing yield and selectivity, namely catalyst, coordinating ligand, and solvent, are detailed.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service