454370
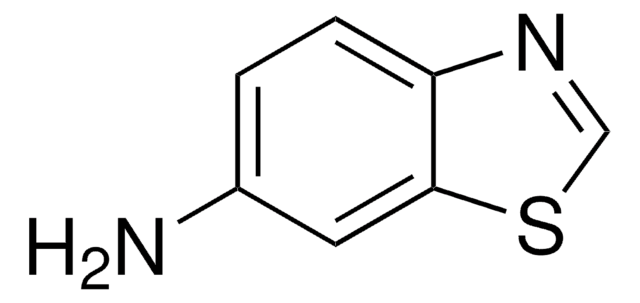
6-Amino-2-mercaptobenzothiazole
97%
Synonym(s):
6-Amino-2-benzothiazolethiol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H6N2S2
CAS Number:
Molecular Weight:
182.27
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
267-272 °C (lit.)
SMILES string
Nc1ccc2nc(S)sc2c1
InChI
1S/C7H6N2S2/c8-4-1-2-5-6(3-4)11-7(10)9-5/h1-3H,8H2,(H,9,10)
InChI key
IDPNFKLUBIKHSW-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
[A QSAR study of synthetic stimulators of growth of domestic animals on the basis of 6-amino-2-mercaptobenzothiazole].
P Zahradník et al.
Ceskoslovenska farmacie, 37(10), 440-442 (1988-12-01)
Synthesis and antimicrobial activity of 2-alkylthio-6-aminobenzothiazoles.
Sidoova E, et al.
Chemical Papers, 33, 830-836 (1979)
Electrochemical Characterization of Gold 6-Amino-2-mercaptobenzothiazole Self-Assembled Monolayer for Dopamine Detection in Pharmaceutical Samples.
Shervedani RK, et al.
Electroanalysis, 22(9), 969-977 (2010)
Aiming Sun et al.
ChemMedChem, 6(4), 654-666 (2011-03-03)
Small molecules, namely coactivator binding inhibitors (CBIs), that block estrogen signaling by directly inhibiting the interaction of the estrogen receptor (ER) with coactivator proteins act in a fundamentally different way to traditional antagonists, which displace the endogenous ligand estradiol. To
Triorganotin (IV) complexes of Schiff base derived from 6-amino-2-mercaptobenzothiazole: Synthesis, characterization and X-ray crystal structures.
Ma C, et al.
Inorgorganica Chimica Acta, 360(7), 2439-2446 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service