All Photos(1)
About This Item
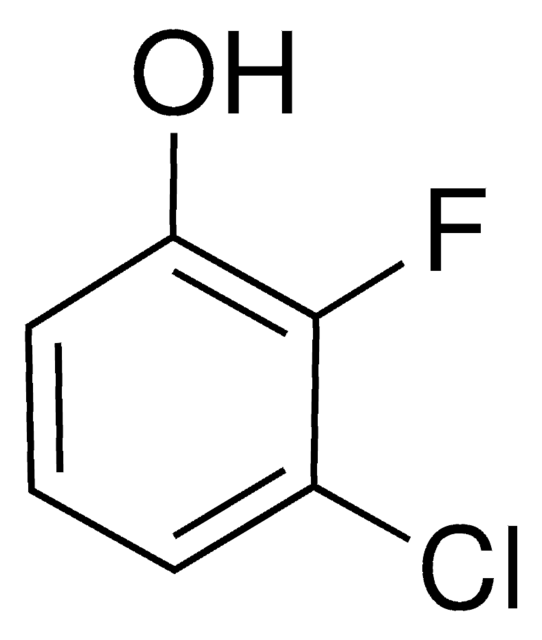
Linear Formula:
ClC6H3(F)OH
CAS Number:
Molecular Weight:
146.55
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
bp
84 °C/44 mmHg (lit.)
mp
54-56 °C (lit.)
solubility
chloroform: soluble 50 mg/mL, clear, colorless
SMILES string
Oc1ccc(Cl)c(F)c1
InChI
1S/C6H4ClFO/c7-5-2-1-4(9)3-6(5)8/h1-3,9H
InChI key
XLHYAEBESNFTCA-UHFFFAOYSA-N
General description
4-Chloro-3-fluorophenol is hydroxylated at both ortho positions to yield different products.
Application
4-Chloro-3-fluorophenol was used in the synthesis of 4-chloro-3-fluoro catechol.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
L Ridder et al.
European journal of biochemistry, 257(1), 92-100 (1998-11-03)
The influence of various C4/C5 substituents in catechol (1,2-dihydroxybenzene) derivatives on the overall rate of conversion by catechol-1,2-dioxygenase from Pseudomonas putida (arvilla) C1 was investigated. Using catechol, 4-methylcatechol, 4-fluorocatechol, 4-chlorocatechol, 4-bromocatechol, 4,5-difluorocatechol and 4-chloro-5-fluorocatechol, it could be demonstrated that substituents
S Peelen et al.
European journal of biochemistry, 227(1-2), 284-291 (1995-01-15)
This study describes the regioselective hydroxylation and the rates of conversion of a series of fluorinated phenol derivatives by phenol hydroxylase from the yeast Trichosporon cutaneum. The natural logarithm of the kcat value for the conversion of the phenolic substrates
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service