All Photos(3)
About This Item
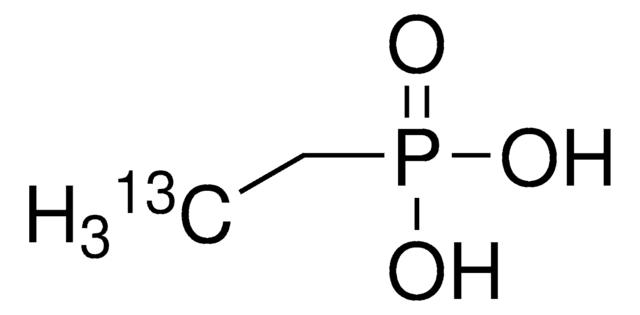
Linear Formula:
CH3P(O)(OH)2
CAS Number:
Molecular Weight:
96.02
Beilstein:
1739372
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
105-107 °C (lit.)
SMILES string
CP(O)(O)=O
InChI
1S/CH5O3P/c1-5(2,3)4/h1H3,(H2,2,3,4)
InChI key
YACKEPLHDIMKIO-UHFFFAOYSA-N
Gene Information
human ... ALPP(250)
Looking for similar products? Visit Product Comparison Guide
General description
The vibrational spectra of aqueous solutions of methylphosphonic acid and its anions were studied. The supercritical water co-oxidation elementary reaction rate mechanism for methylphosphonic acid was also studied.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 2
Flash Point(F)
>392.0 °F - Pensky-Martens closed cup
Flash Point(C)
> 200 °C - Pensky-Martens closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Vibrational analysis of methylphosphonic acid and its anions: I. Vibrational spectra.
Journal of Molecular Structure, 15(2), 225-236 (1973)
Co-oxidation of methylphosphonic acid and ethanol in supercritical water: II: Elementary reaction rate model.
Journal of Supercritical Fluids, 39(2), 239-245 (2006)
Janel Owens et al.
Journal of agricultural and food chemistry, 57(18), 8227-8235 (2009-08-19)
Though chemical warfare agents (CWAs) have been banned by the Chemical Weapons Convention, the threat that such chemicals may be used, including their deliberate addition to food, remains. In such matrixes, CWAs may hydrolyze to phosphonic acids, which are good
Guoqiang Feng et al.
Journal of the American Chemical Society, 131(35), 12771-12779 (2009-08-14)
Reactivities of five phosphonate esters each coordinated to a dinuclear Co(III) complex were investigated ([Co(2)(tacn)(2)(OH)(2){O(2)P(Me)OAr}](3+); tacn = 1,4,7-triazacyclononane; substituent = m-F, p-NO(2) (1a); p-NO(2) (1b); m-NO(2) (1c); p-Cl (1d); unsubstituted (1e)). Hydrolysis of the phosphonate esters in 1a to 1e
J Lützenkirchen et al.
Advances in colloid and interface science, 157(1-2), 61-74 (2010-05-11)
A tentative picture for the charging of the sapphire basal plane in dilute electrolyte solutions allows reconciliation of the available experimental observations within a dual charging model. It includes the MUltiSIte Complexation (MUSIC) model and auto-protolysis of interfacial water. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service