All Photos(1)
About This Item
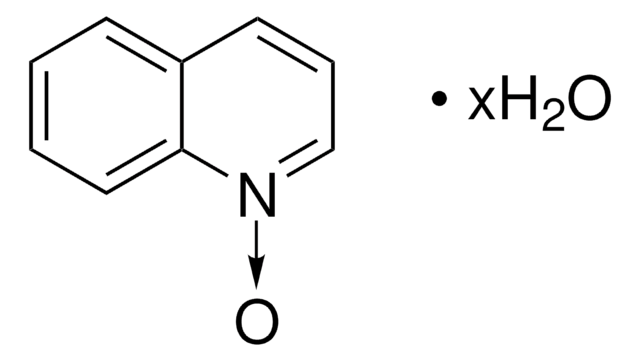
Empirical Formula (Hill Notation):
C9H7NO
CAS Number:
Molecular Weight:
145.16
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
103-105 °C (lit.)
SMILES string
[O-][n+]1ccc2ccccc2c1
InChI
1S/C9H7NO/c11-10-6-5-8-3-1-2-4-9(8)7-10/h1-7H
InChI key
RZIAABRFQASVSW-UHFFFAOYSA-N
General description
Photochemical isomerization of isoquinoline N-oxide in methanol or water has been investigated by steady-light irradiation and flash spectroscopy. It is a useful intermediate for isoquinoline derivatives. It causes the oxidation of fullerene C60 under ultrasonic irradiation in air.
Application
Isoquinoline N-oxide was used in the synthesis of N-alkoxy isoquinolinium and N-alkoxy 4-phenylpyridinium ion terminated polystyrenes.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Iodine-mediated electrophilic cyclization of 2-alkynylbenzaldoximes leading to the formation of iodoisoquinoline N-oxides.
Huo Z, et al.
Tetrahedron Letters, 49(38), 5531-5533 (2008)
The primary photochemical process of isoquinoline N-oxide in hydroxylic solvents.
Ono I and Hata N.
Bulletin of the Chemical Society of Japan, 46, 3658-3662 (1973)
N-alkoxy pyridinium ion terminated polystyrenes: A facile route to photoinduced block copolymerization.
Durmaz YY, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 45(3), 423-428 (2007)
Weon-Bae Ko et al.
Ultrasonics, 42(1-9), 611-615 (2004-03-30)
The reaction of C60 with various amine N-oxides such as 3-picoline N-oxide (Aldrich 98.0%), pyridine N-oxide hydrate (Aldrich 95.0%), quinoline N-oxide (Aldrich 97.0%), isoquinoline N-oxide (Aldrich 98.0%) under ultrasonic irradiation in air at 25-43 degrees C causes the oxidation of
Oleg V Larionov et al.
Organic letters, 16(3), 864-867 (2014-01-15)
A one-step transformation of heterocyclic N-oxides to 2-alkyl-, aryl-, and alkenyl-substituted N-heterocycles is described. The success of this broad-scope methodology hinges on the combination of copper catalysis and activation by lithium fluoride or magnesium chloride. The utility of this method
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service