All Photos(1)
About This Item
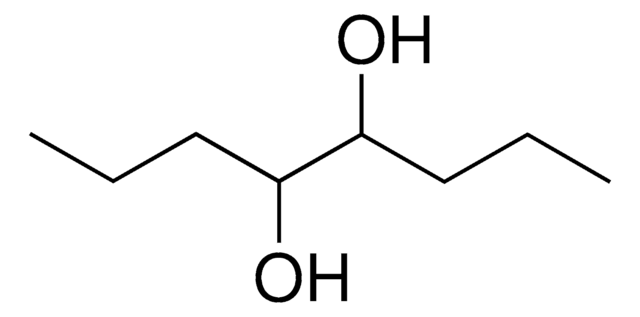
Linear Formula:
HOCH2C[(CH2)3CH3](C2H5)CH2OH
CAS Number:
Molecular Weight:
160.25
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
bp
178 °C/50 mmHg (lit.)
mp
41-44 °C (lit.)
functional group
hydroxyl
SMILES string
CCCCC(CC)(CO)CO
InChI
1S/C9H20O2/c1-3-5-6-9(4-2,7-10)8-11/h10-11H,3-8H2,1-2H3
InChI key
DSKYSDCYIODJPC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Butyl-2-ethyl-1,3-propanediol undergoes bulk polycondensation with diacid monomer (terephthalic acid) to yield poly(ethylene terephthalate) copolymers.
Application
2-Butyl-2-ethyl-1,3-propanediol was used in the synthesis of polyesters.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point(F)
276.8 °F - closed cup
Flash Point(C)
136 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Structural characterization and thermal properties of poly (ethylene terephthalate) copolymers containing 2-butyl-2-ethyl-1, 3-propanediol.
Kint DPR, et al.
Journal of Applied Polymer Science, 86(5), 1077-1086 (2002)
Olli Laine et al.
Analytical chemistry, 74(16), 4250-4258 (2002-08-30)
Polyesters prepared from the same diol, 2-butyl-2-ethyl-1,3-propanediol, but different phthalic acid isomers, phthalic, isophthalic, and terephthalic acid, were characterized by collision-induced dissociation electrospray ionization Fourier transform ion cyclotron resonance (CID-ESI-FT-ICR) and postsource-decay matrix-assisted laser desorption/ionization time-of-flight (PSD-MALDI-TOF) mass spectrometry. Sodiated
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service