F9813
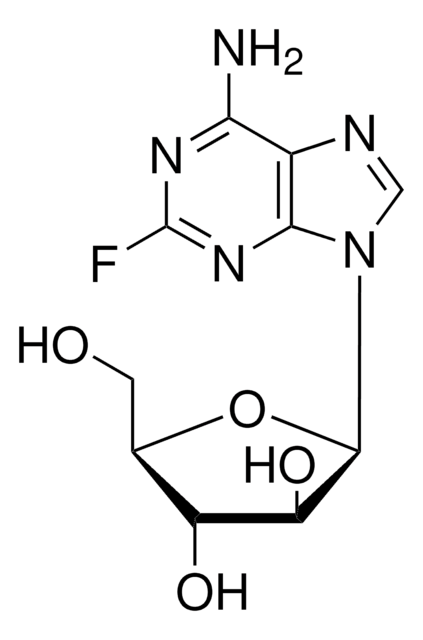
Fludarabine phosphate
Synonym(s):
2-Fluoro-9-(5-O-phosphono-β-D-arabinofuranosyl)-9H-purin-6-amine
About This Item
Recommended Products
form
powder
Quality Level
color
white
solubility
DMSO: soluble
antibiotic activity spectrum
neoplastics
Mode of action
DNA synthesis | interferes
storage temp.
−20°C
InChI
1S/C10H13FN5O7P/c11-10-14-7(12)4-8(15-10)16(2-13-4)9-6(18)5(17)3(23-9)1-22-24(19,20)21/h2-3,5-6,9,17-18H,1H2,(H2,12,14,15)(H2,19,20,21)
InChI key
GIUYCYHIANZCFB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Characterization of Chemical Interactions between Clinical Drugs and the Oral Bacterium, Corynebacterium matruchotii, via Bioactivity-HiTES.: This study explores the interactions of clinical drugs like Fludarabine phosphate with Corynebacterium matruchotii, highlighting potential impacts on oral microbiota and implications for drug efficacy and safety (Lee DY et al., 2024).
- Cocktail of lipophilic and hydrophilic chemotherapeutics in high-load core@shell nanocarriers to treat pancreatic tumours.: Investigates the efficacy of a combination of Fludarabine phosphate with other chemotherapeutics delivered via nanocarriers, aiming to enhance treatment outcomes for pancreatic cancer by improving drug delivery to the tumor site (Rudolph D et al., 2024).
- Macrophage neogenin deficiency exacerbates myocardial remodeling and inflammation after acute myocardial infarction through JAK1-STAT1 signaling.: This research demonstrates the role of Fludarabine phosphate in modulating inflammation and cardiac repair post-myocardial infarction, offering insights into its potential therapeutic benefits beyond oncology (Zhang J et al., 2023).
- SLC25A51 promotes tumor growth through sustaining mitochondria acetylation homeostasis and proline biogenesis.: Discusses the cellular mechanisms by which Fludarabine phosphate may influence metabolic pathways in cancer cells, highlighting its potential to disrupt tumor metabolism and promote cancer cell death (Li Y et al., 2023).
- CD19-Targeting CAR T Cells for Myositis and Interstitial Lung Disease Associated With Antisynthetase Syndrome.: Reviews the use of Fludarabine phosphate in preconditioning regimens for CAR T-cell therapy, emphasizing its role in enhancing the efficacy of immunotherapy in treating autoimmune disorders (Pecher AC et al., 2023).
Biochem/physiol Actions
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Muta. 2 - Repr. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service