All Photos(1)
About This Item
Empirical Formula (Hill Notation):
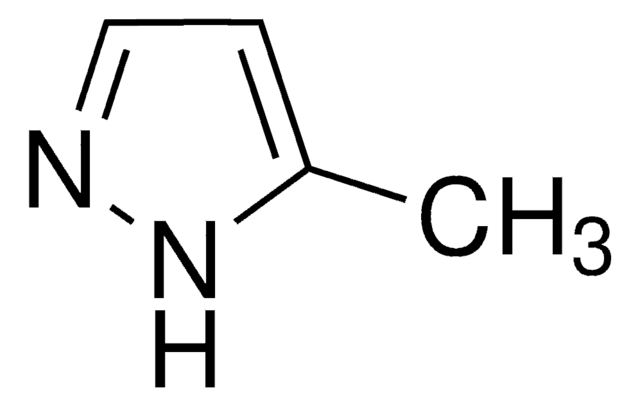
C4H6N2
CAS Number:
Molecular Weight:
82.10
Beilstein:
1454
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.495 (lit.)
bp
204 °C (lit.)
density
1.02 g/mL at 25 °C (lit.)
SMILES string
Cc1cc[nH]n1
InChI
1S/C4H6N2/c1-4-2-3-5-6-4/h2-3H,1H3,(H,5,6)
InChI key
XKVUYEYANWFIJX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Repr. 1B - Skin Corr. 1B - STOT RE 2
Target Organs
Lungs
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Flash Point(F)
218.3 °F - closed cup
Flash Point(C)
103.5 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
B Schulz et al.
Die Pharmazie, 41(2), 118-120 (1986-02-01)
The diffusion of 3-methylpyrazole through a synthetic polymer matrix and the effect of the solubility of the bioactive agent in polymers on the release behaviour of polymer combinations were studied. With increasing hydrophilicity of the polymer both the diffusion and
B Schulz et al.
Die Pharmazie, 43(1), 29-31 (1988-01-01)
The release behaviour of the antimicrobially active 3(5)-methylpyrazole from matrix systems prepared from maleic anhydride copolymers as well as from copolymers of maleic esters and maleic amides was studied. Under alkaline conditions erosion is the predominant release mechanism compared to
B Schulz et al.
Die Pharmazie, 40(8), 548-552 (1985-08-01)
The release of 3-methylpyrazole from monolithic polymer films into aqueous media has been studied. The diffusion of the active agent decreased with increasing of the content of acetate groups in reacetylated poly(vinyl alcohols) and with increasing of the ester lengths
E S Fiala et al.
Journal of cancer research and clinical oncology, 113(2), 145-150 (1987-01-01)
Using a hybrid ion-exchange reverse phase HPLC system, we found that F344 rat liver microsomes, in the presence of an NADPH-generating system, can metabolize methylazoxymethanol (MAM), a colon and liver carcinogen, to methanol and formic acid. This is in contrast
[Toxicological evaluation of a new nitrification inhibitor and its metabolite].
S B Pogosian et al.
Gigiena i sanitariia, (6)(6), 29-31 (1986-06-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service