C94009
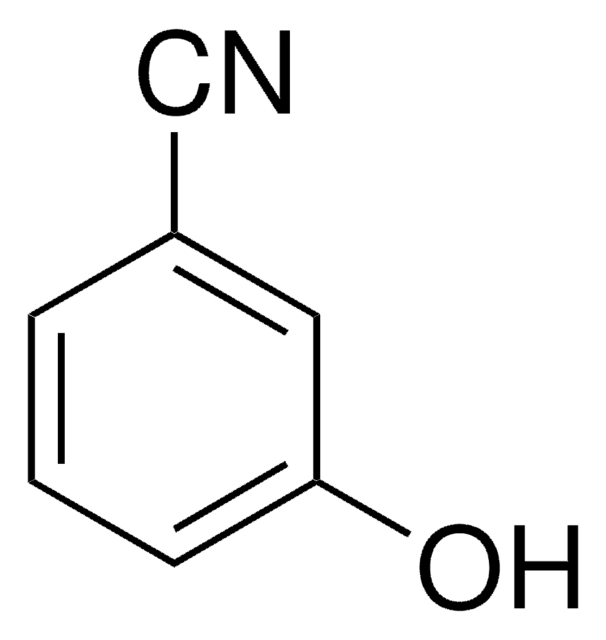
4-Cyanophenol
95%
Synonym(s):
4-Hydroxybenzonitrile
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
NCC6H4OH
CAS Number:
Molecular Weight:
119.12
Beilstein:
386130
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
crystals
mp
110-113 °C (lit.)
SMILES string
Oc1ccc(cc1)C#N
InChI
1S/C7H5NO/c8-5-6-1-3-7(9)4-2-6/h1-4,9H
InChI key
CVNOWLNNPYYEOH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
4-Cyanophenol is a precursor for the synthesis of a vasodilator, Levcromakalim. Bromination of 4-cyanophenol results in bromoxynil, a commercial herbicide. It can also be used as a component of deep eutectic solvent (DES) mixture.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Nitrogen-doped carbons prepared from eutectic mixtures as metal-free oxygen reduction catalysts.
Lopez-Salas N, et al.
Journal of Material Chemistry A, 4(2), 478-488 (2016)
Synthesis and antihypertensive activity of 4-(cyclic amido)-2H-1-benzopyrans.
Ashwood VA, et al.
Journal of Medicinal Chemistry, 29(11), 2194-2201 (1986)
D B Harper
The International journal of biochemistry, 17(6), 677-683 (1985-01-01)
The purification and properties of an enzyme from Nocardia sp. which catalyses the conversion of p-hydroxybenzonitrile to p-hydroxybenzoic acid and ammonia without intermediate formation of the amide is described. The enzyme displayed a broad pH optimum between 7.0 and 9.5
A L Stinchcomb et al.
Pharmaceutical research, 16(8), 1288-1293 (1999-09-01)
Simple, safe and quick in vivo methods for estimating chemical uptake into the stratum corneum (SC) from volatile and non-volatile solvents are invaluable to health risk assessors. This study compares the human in vivo SC uptake of a model compound
High atom efficient and environment-friendly preparation of herbicides bromoxynil and ioxynil.
Joshi G and Patil RD
Indian Journal of Chemistry, 49B, 1678-1680 (2010)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service