272361
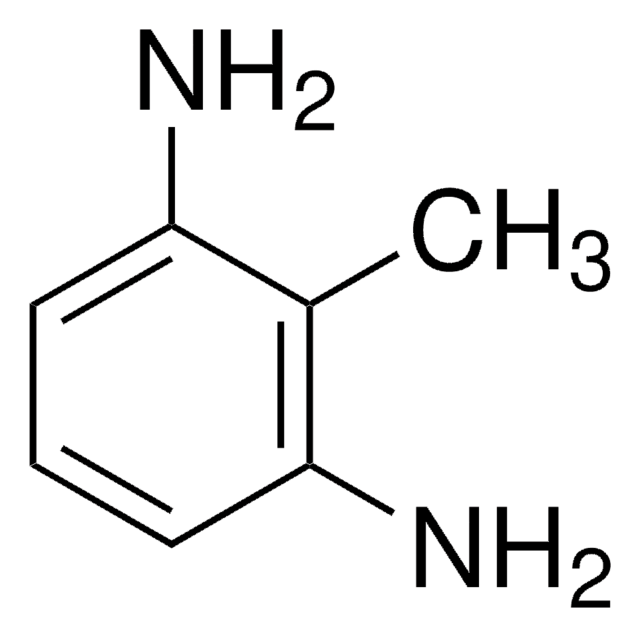
2,3-Diaminotoluene
97%
Synonym(s):
3-Methyl-o-phenylenediamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3C6H3(NH2)2
CAS Number:
Molecular Weight:
122.17
Beilstein:
907184
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
59-65 °C (lit.)
SMILES string
Cc1cccc(N)c1N
InChI
1S/C7H10N2/c1-5-3-2-4-6(8)7(5)9/h2-4H,8-9H2,1H3
InChI key
AXNUJYHFQHQZBE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
The antitumour activity of Pd(II) and Pt(II) new complexes with 2,3-diaminotoluene was studied. 2,3-Diaminotoluene induced CYP1A activity.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M Pérez-Cabré et al.
Journal of inorganic biochemistry, 98(3), 510-521 (2004-02-28)
Pd(II) and Pt(II) new complexes with simple aromatic diamines were synthesised and characterised with the aim of studying their possible antitumour activity. The aromatic diamines chosen were 2,3-diaminotoluene (2,3 dat), 3,4-diaminotoluene (3,4 dat), 4,5-diaminoxylene (4,5 dax) and 2,3-diaminophenol (2,3 dap).
Y L Cheung et al.
Toxicology and applied pharmacology, 139(1), 203-211 (1996-07-01)
The present study was undertaken to provide a rationale for the marked difference in carcinogenic potential among isomeric diaminotoluenes, in relation to their ability to induce their own bioactivation through CYP1A induction, their genotoxic potential, and their ability to bind
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service