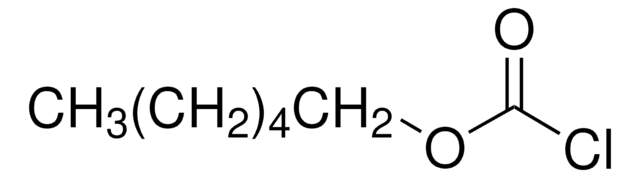185892
Ethyl chloroformate
97%
Synonym(s):
Chloroformic acid ethyl ester
About This Item
Recommended Products
vapor density
3.74 (vs air)
Quality Level
vapor pressure
3.42 psi ( 20 °C)
Assay
97%
form
liquid
autoignition temp.
842 °F
refractive index
n20/D 1.395 (lit.)
bp
93 °C (lit.)
mp
−81 °C (lit.)
solubility
alcohol: miscible
benzene: miscible
chloroform: miscible
diethyl ether: miscible
water: insoluble
density
1.135 g/mL at 25 °C (lit.)
SMILES string
CCOC(Cl)=O
InChI
1S/C3H5ClO2/c1-2-6-3(4)5/h2H2,1H3
InChI key
RIFGWPKJUGCATF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Ethyl chloroformate was used in the synthesis of nitrile oxides.
- It was used as derivatization reagent to investigate stereochemical conversion of chiral non-steroidal anti-inflammatory drugs during derivatization reaction.
- It was used to develop flexible template strategy for the generation of nanometer-scale templates via dip-pen nanolithography.
- It was used as derivatization reagent in an enantiomeric analysis of amino acids by two-dimensional gas chromatography.
- It was also used with ammonia and cyanuric chloride to convert carboxylic acids to nitriles.
Packaging
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Met. Corr. 1 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
60.8 °F
Flash Point(C)
16 °C
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service