T9299
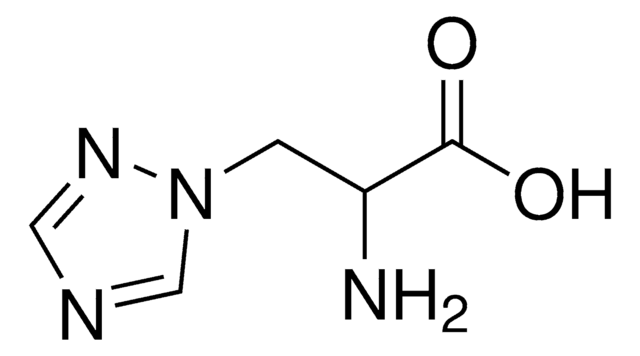
β-(1,2,4-Triazol-3-yl)-DL-alanine
≥98% (TLC)
Synonym(s):
(±)-2-Amino-3-(1,2,4-triazol-3-yl)propionic acid, DL-1,2,4-Triazole-3-alanine
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C5H8N4O2
CAS Number:
Molecular Weight:
156.14
EC Number:
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
β-(1,2,4-Triazol-3-yl)-DL-alanine,
Assay
≥98% (TLC)
Quality Level
form
powder
color
white to off-white
storage temp.
−20°C
SMILES string
NC(Cc1nc[nH]n1)C(O)=O
InChI
1S/C5H8N4O2/c6-3(5(10)11)1-4-7-2-8-9-4/h2-3H,1,6H2,(H,10,11)(H,7,8,9)
InChI key
CAPORZWUTKSILW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Linkage
Inhibitory histidine analog.
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
D E Levin et al.
Environmental mutagenesis, 8(1), 9-28 (1986-01-01)
The standard Salmonella tester strains used to detect base substitution mutations carry the hisG428 ochre mutation (TA102 and TA104) and the hisG46 missense mutation (TA100). These mutations can be reverted by base changes at their mutant his loci or at
Strauss, A., et al.
Plant Cell, Tissue and Organ Culture, 3, 123-123 (1984)
S H Beiboer et al.
Protein engineering, 9(4), 345-352 (1996-04-01)
The effect of the substitution of the active site histidine 48 by the unnatural 1,2,4-triazole-3-alanine (TAA) amino acid analogue in porcine pancreas phospholipase A2 (PLA2) was studied. TAA was introduced biosynthetically using a his-auxotrophic Escherichia coli strain. To study solely
Younas Aouine et al.
Molecules (Basel, Switzerland), 16(4), 3380-3390 (2011-04-23)
A simple synthetic approach to racemic N-tert-butyloxycarbonyl-2-methyl-3-(1H-1,2,4-triazol-1-yl)alanine (5) in four steps and 68% overall yield starting from oxazoline derivative 1 is reported. This synthesis involves the alkylation of 1H-1,2,4-triazole with an O-tosyloxazoline derivative, followed by an oxazoline ring-opening reaction and
K A Sment et al.
Applied and environmental microbiology, 55(5), 1295-1297 (1989-05-01)
In contrast to wild-type cells, it was found that triazole-alanine-resistant mutants of Methanococcus voltae excreted histidine, proline, phenylalanine, and tyrosine in various combinations. These results suggest that a form of general amino acid biosynthetic control may operate in this methanogen.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service