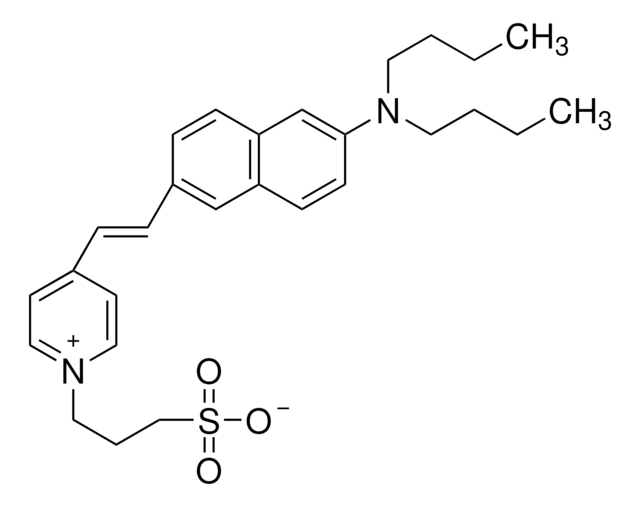
I5773
IDRA 21
≥98%
Synonym(s):
7-Chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H9ClN2O2S
CAS Number:
Molecular Weight:
232.69
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥98%
SMILES string
CC1Nc2ccc(Cl)cc2S(=O)(=O)N1
InChI
1S/C8H9ClN2O2S/c1-5-10-7-3-2-6(9)4-8(7)14(12,13)11-5/h2-5,10-11H,1H3
InChI key
VZRNTCHTJRLTMU-UHFFFAOYSA-N
Biochem/physiol Actions
Blocks the rapid desensitization of the AMPA receptors and markedly prolongs the decay time of the evoked excitatory post-synaptic current.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Gabriele Losi et al.
Neuropharmacology, 46(8), 1105-1113 (2004-04-28)
IDRA-21 (7-chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxide) reduces alpha-amino-3-hydroxy-5-methylisoxazolepropionic acid (AMPA) receptors desensitisation in vitro and restores learning and cognitive impairment in vivo. In this study, we show that in cerebellar granule cells (CGCs) in culture IDRA-21 reduces N-methyl-d-aspartate receptor (NMDAR) whole-cell currents. The
D P Uzunov et al.
Journal of pharmaceutical sciences, 84(8), 937-942 (1995-08-01)
The direct analytical and semipreparative high-performance liquid chromatographic (HPLC) resolution of the enantiomers of IDRA 21 [1,7-chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine S,S-dioxide] is reported. (+/-)-IDRA 21 administered orally to rats subjected to a water maze cognition test elicited a performance enhancing effect. Between the
G Cannazza et al.
Journal of pharmaceutical and biomedical analysis, 23(1), 117-125 (2000-07-18)
Analytical high-performance liquid chromatography (HPLC) methods using derivatized cellulose chiral stationary phases (CSPs) were developed for the separation of the enantiomers of 7-chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxide ((+/-) IDRA21). In previous studies, (+/-) IDRA21 has been found to have an interesting inhibitory effect
G Puia et al.
Progress in neuro-psychopharmacology & biological psychiatry, 24(6), 1007-1015 (2000-10-21)
1. Patch-clamp technique was used in primary cultures of cerebellar granule neurons to study the modulation of the cyclothiazide analogue (IDRA21) and of the diazoxide derivative (IDRA 5) on KA-evoked currents. 2. The dose-response of kainic acid (KA) reveals an
K A Yamada et al.
Neurobiology of disease, 5(3), 196-205 (1998-12-16)
The diazoxide derivative 7-chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-S,S-dioxide (IDRA21) enhances memory and learning in rodents, most likely by potentiating AMPAergic synaptic activity. We examined IDRA21's effect upon AMPAergic synaptic currents and whole-cell glutamate currents in cultured rat hippocampal neurons to determine whether IDRA21 was
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service