901546
Poly(M(EO)2MA:Poly(OEGMA) 90:10
hydrazide functionalized, 25 wt. % (solution in water)
Synonym(s):
PEGMA-co-POEGMA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
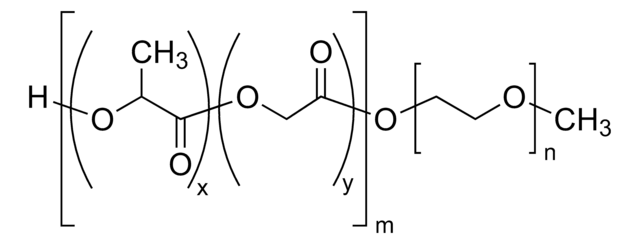
[CH2CCH3(COO(CH2CH2O)2CH3)]p[CH2CCH3(COO(CH2CH2O)8CH3)]n[CH2CHCONHNHCO(CH2)4CONHNH2]m
UNSPSC Code:
12162002
NACRES:
NA.23
Recommended Products
form
solution
mol wt
Mw ~20,000 g/mol
concentration
25 wt. % (solution in water)
color
clear colorless to pale yellow
functional group
hydrazide
storage temp.
2-8°C
Application
In addition, the physical properties of the resulting hydrogels, such as LCST, gelation rates, swelling kinetics, degradation kinetics, and mechanical properties, can all be readily controlled by solution concentration and the ratios of each solution.
This product is provided as a 25 wt% solution in water, ready to be diluted for your specific application. Please see the technical bulletin on the product page for dilution instructions and hydrogel preparation instructions.
This product is provided as a 25 wt% solution in water, ready to be diluted for your specific application. Please see the technical bulletin on the product page for dilution instructions and hydrogel preparation instructions.
Poly(diethylene glycol methacrylate)-co-(oligoethylene glycol methacrylate), (PM(EO)2MA-co-OEGMA) is a comb-shaped, graft copolymer consisting of hydrophilic oligomer polyethylene glycol (PEG) chains grafted to a hydrophobic polymethacrylate backbone. In this material, the PEG chains are either diethylene glycol units or oligomer PEG (n=8-9) units. Resulting material properties can be tuned by controlling the ratio of each component. POEGMA has been suggested as a viable alternative to PEG in biological and biomaterial applications. POEGMA has been reported to improve pharmacokinetic properties of protein and peptide conjugates, enhance the stability and gene silencing efficiency of siRNAs, as an anti-fouling surface for biosensors, and eliminate PEG antigenicity. In addition to use in biomolecule-polymer conjugates, PEOGMA has also seen wide spread use in tissue engineering applications, such as hydrogel synthesis. Hydrazide-functionalized PM(EO)2MA-co-OEGMA can be readily used with the corresponding aldehyde-functionalized POEGMA or PM(EO)2MA-co-OEGMA for rapid gelation via reversible hydrazone bond formation. Due to the reversibility of the bond formation and the low viscosity of the precursors, resulting hydrogels can be used as injectable tissue engineering matrices, local drug delivery vehicles for small molecules, or as joint lubricants.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A brush-polymer/exendin-4 conjugate reduces blood glucose levels for up to five days and eliminates poly(ethylene glycol) antigenicity.
Qi Y, et al.
Nature Biomedical Engineering, 1, 0002-0002 (2016)
Reactive electrospinning of degradable poly(oligoethylene glycol methacrylate)-based nanofibrous hydrogel networks.
Xu F, et al.
Chemical Communications (Cambridge, England), 52, 1451-1454 (2016)
Weiping Gao et al.
Proceedings of the National Academy of Sciences of the United States of America, 106(36), 15231-15236 (2009-08-27)
The challenge in the synthesis of protein-polymer conjugates for biological applications is to synthesize a stoichiometric (typically 1:1) conjugate of the protein with a monodisperse polymer, with good retention of protein activity, significantly improved pharmacokinetics and increased bioavailability, and hence
Imran Ozer et al.
Biomacromolecules, 18(9), 2699-2710 (2017-08-05)
PEGylation, covalent attachment of PEG to therapeutic biomolecules, in which suboptimal pharmacokinetic profiles limiting their therapeutic utility are of concern, is a widely applied technology. However, this technology has been challenged by reduced bioactivity of biomolecules upon PEGylation and immunogenicity
Niels M B Smeets et al.
Chemical communications (Cambridge, England), 50(25), 3306-3309 (2014-02-18)
Injectable PEG-analogue hydrogels based on poly(oligoethylene glycol methacrylate) have been developed based on complementary hydrazide and aldehyde reactive linear polymer precursors. These hydrogels display the desired biological properties of PEG, form covalent networks in situ following injection, and are easily
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service