89231
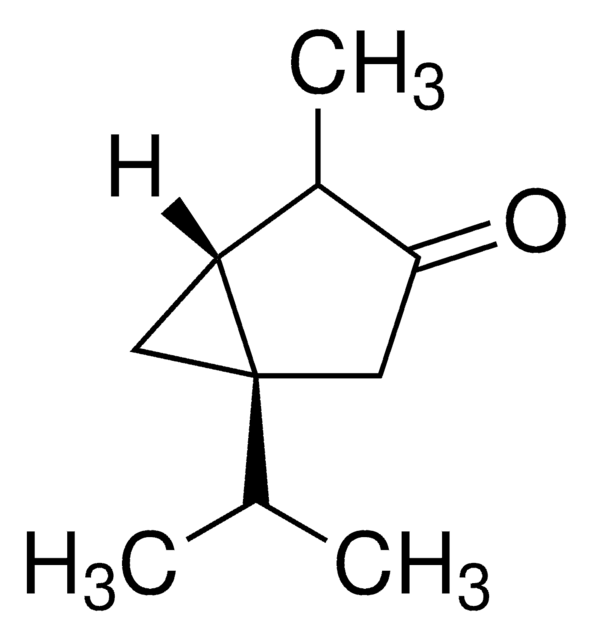
(−)-α-Thujone
≥96.0% (GC)
Synonym(s):
(-)-alpha-Thujone, (1S,4R)-1-Isopropyl-4-methylbicyclo[3.1.0]hexan-3-one
About This Item
Recommended Products
Assay
≥96.0% (GC)
form
liquid
optical activity
[α]20/D −19.0±2.0°, neat
refractive index
n20/D 1.450
density
0.914 g/mL at 20 °C (lit.)
storage temp.
2-8°C
SMILES string
CC(C)[C@@]12C[C@@H]1[C@@H](C)C(=O)C2
InChI
1S/C10H16O/c1-6(2)10-4-8(10)7(3)9(11)5-10/h6-8H,4-5H2,1-3H3/t7-,8-,10+/m1/s1
InChI key
USMNOWBWPHYOEA-MRTMQBJTSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- A six-step total synthesis of α-thujone and d6-α-thujone, enabling facile access to isotopically labelled metabolites: This study presents a concise method for synthesizing α-thujone, useful for creating isotopically labeled derivatives for research purposes (Thamm et al., 2016).
- Enhancement of CD3AK cell proliferation and killing ability by α-thujone: Investigates α-thujone′s ability to enhance the proliferation and cytotoxic activity of CD3AK cells, suggesting potential immunological applications (Zhou et al., 2016).
- α-Thujone exhibits an antifungal activity against F. graminearum by inducing oxidative stress, apoptosis, epigenetics alterations and reduced toxin synthesis: Demonstrates the antifungal effects of α-thujone against Fusarium graminearum, which could make it a valuable alternative to traditional fungicides (Teker et al., 2021).
Biochem/physiol Actions
Other Notes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
147.2 °F - closed cup
Flash Point(C)
64 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service