665118
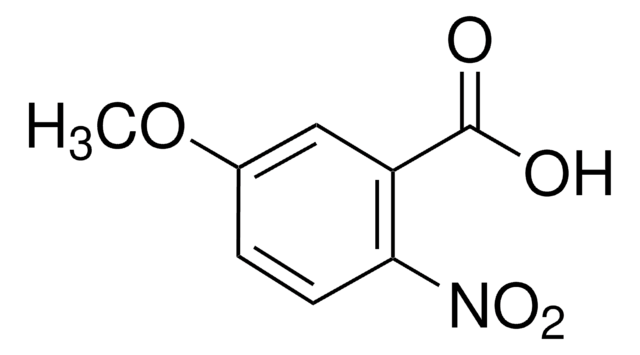
2-Amino-5-methoxybenzoic acid
97%
Synonym(s):
6-Amino-m-anisic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(H2N)C6H3(OCH3)CO2H
CAS Number:
Molecular Weight:
167.16
MDL number:
UNSPSC Code:
12352106
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
reaction suitability
reaction type: solution phase peptide synthesis
mp
148-152 °C
application(s)
peptide synthesis
SMILES string
COc1ccc(N)c(c1)C(O)=O
InChI
1S/C8H9NO3/c1-12-5-2-3-7(9)6(4-5)8(10)11/h2-4H,9H2,1H3,(H,10,11)
InChI key
UMKSAURFQFUULT-UHFFFAOYSA-N
Application
2-Amino-5-methoxybenzoic acid is a general reagent used in the synthesis of substituted isoquinolinonaphthyridines, quinazolinones, imidazobenzodiazepines, pyridoquinazolones and polycyclic hexahydrobenzo[c]acridines.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Asymmetric synthesis of isoquinolinonaphthyridines catalyzed by a chiral Br?nsted acid.
Li J, et al.
Organic & Biomolecular Chemistry, 15(31), 6474-6477 (2017)
Bismuth (III) Chloride Catalyzed Intramolecular Hetero-Diels?Alder Reaction: Access to cis-Fused Angular Hexahydrobenzo[c]acridines.
Sabitha G, et al.
Synthesis, 47(01), 124-128 (2015)
Condensation of anthranilic acids with pyridines to furnish pyridoquinazolones via pyridine dearomatization.
Yang Y, et al.
Chemical Communications (Cambridge, England), 52(87), 12869-12872 (2016)
Biologically active heterocyclic hybrids based on quinazolinone, benzofuran and imidazolium moieties: synthesis, characterization, cytotoxic and antibacterial evaluation.
Asadi P, et al.
Chemistry and Biodiversity, 14(4), e1600411-e1600411 (2017)
Optimization of substituted imidazobenzodiazepines as novel asthma treatments.
Jahan R, et al.
European Journal of Medicinal Chemistry, 126, 550-560 (2017)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service