160695
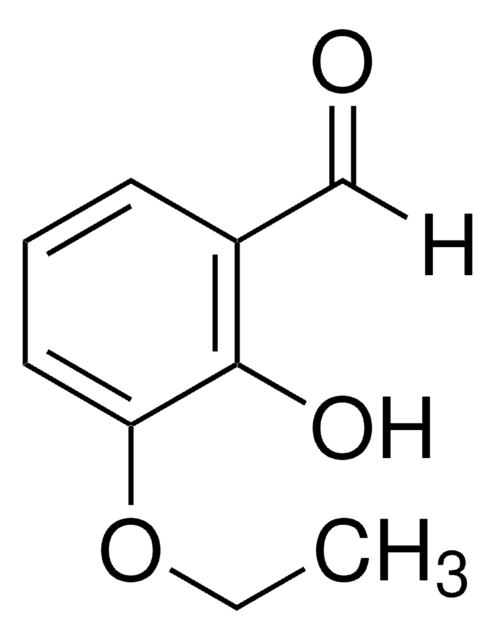
2-Hydroxy-4-methoxybenzaldehyde
98%
Synonym(s):
4-Methoxysalicylaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOC6H3(OCH3)CHO
CAS Number:
Molecular Weight:
152.15
Beilstein:
1072443
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
41-43 °C (lit.)
SMILES string
[H]C(=O)c1ccc(OC)cc1O
InChI
1S/C8H8O3/c1-11-7-3-2-6(5-9)8(10)4-7/h2-5,10H,1H3
InChI key
WZUODJNEIXSNEU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Hydroxy-4-methoxybenzaldehyde is the main component of root bark essential oil of Periploca sepium Bunge. It is a potential tyrosinase inhibitor present in African medicinal plants.
Application
2-Hydroxy-4-methoxybenzaldehyde was used in the synthesis of Schiff base ligand.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Gholamreza Karimipour et al.
Biological trace element research, 145(1), 109-117 (2011-08-13)
In this study, a new sorbent based on the gold nanoparticle loaded in activated carbon (Au-NP-AC) was synthesized and modified by bis(4-methoxy salicylaldehyde)-1,2-phenylenediamine (BMSAPD). This sorbent, which is abbreviated as Au-NP-AC-BMSAPD, has been applied for the enrichment and preconcentration of
Ken-ichi Nihei et al.
Bioorganic & medicinal chemistry letters, 14(3), 681-683 (2004-01-27)
Chamaecin (2-hydroxy-4-isopropylbenzaldehyde) was synthesized and tested for its tyrosinase inhibitory activity. It partially inhibits the oxidation of L-3,4-dihydroxyphenylalanine (L-DOPA) catalyzed by mushroom tyrosinase with an IC(50) of 2.3 microM. The inhibition kinetics analyzed by Dixon plots found that chamaecin is
Dipjyoti Chakraborty et al.
Journal of plant physiology, 165(10), 1033-1040 (2007-11-21)
The fragrant rootstocks of Hemidesmus indicus are known to accumulate 2-hydroxy-4-methoxybenzaldehyde (MBALD), yet, the enzymatic route to this hydroxybenzoate is not known. Therefore, root organs of H. indicus hold promises to unravel the biosynthesis related to this phenolic fragrance. Chitosan
Synthesis, crystal structures and fluorescence properties of two new di-and polynuclear Cd (II) complexes with N2O donor set of a tridentate Schiff base ligand.
Basak S, et al.
Polyhedron, 27(4), 1193-1200 (2008)
Jihua Wang et al.
Molecules (Basel, Switzerland), 15(8), 5807-5817 (2010-08-26)
The root bark essential oil of Periploca sepium Bunge (Asclepiadaceae/ Apocynaceae) obtained by hydrodistillation was investigated by GC and GC-MS. 2-Hydroxy-4-methoxybenzaldehyde was found to be the main component (78.8% of the total) among 17 identified compounds. 2-Hydroxy-4-methoxybenzaldehyde was separated and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service