15395
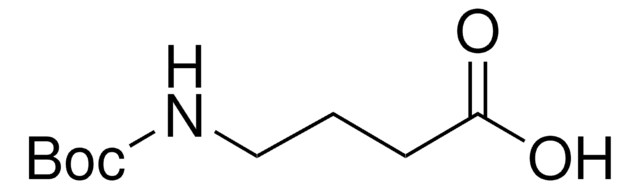
Boc-6-Ahx-OH
≥99.0% (T)
Synonym(s):
6-(Boc-amino)caproic acid, 6-(Boc-amino)hexanoic acid, Boc-6-aminohexanoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)3CO2CNH(CH2)5CO2H
CAS Number:
Molecular Weight:
231.29
Beilstein:
2049561
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99.0% (T)
form
solid
reaction suitability
reaction type: Boc solid-phase peptide synthesis
mp
35-40 °C
application(s)
peptide synthesis
storage temp.
2-8°C
SMILES string
CC(C)(C)OC(=O)NCCCCCC(O)=O
InChI
1S/C11H21NO4/c1-11(2,3)16-10(15)12-8-6-4-5-7-9(13)14/h4-8H2,1-3H3,(H,12,15)(H,13,14)
InChI key
RUFDYIJGNPVTAY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Other Notes
Protected derivative to introduce the ε-aminocaproyl moiety in various compounds. Introduction of an aminocaproyl spacer arm in affinity chromatography.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Affinity chromatography of carboxypeptidase B.
M Sokolovsky
Methods in enzymology, 34, 411-414 (1974-01-01)
A J Schroit et al.
Biochemistry, 22(15), 3617-3623 (1983-07-19)
An efficient method for the synthesis and purification of a variety of iodinated phospholipid analogues is described. 1-Acyl-2-[[[3-(3-[125I]iodo-4-hydroxyphenyl)- propionyl]amino]caproyl]phosphatidylcholine (125I-PC) was prepared by alkylation of 1-acyl-2-(aminocaproyl)phosphatidylcholine with monoiodinated Bolton-Hunter reagent. 125I-Labeled phosphatidic acid, phosphatidylethanolamine, and phosphatidylserine were produced from 125I-PC
The literature on affinity chromatography.
M Wilchek et al.
Methods in enzymology, 34, 3-10 (1974-01-01)
J. Frank et al.
Helvetica Chimica Acta, 60, 2550-2550 (1977)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service