SCP0108
Cathepsin B Substrate
≥95% (HPLC), lyophilized
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C26H36N10O6
Molecular Weight:
584.63
UNSPSC Code:
12352204
NACRES:
NA.32
Recommended Products
product name
Cathepsin B Substrate, colorimetric,
Assay
≥95% (HPLC)
form
lyophilized
composition
Peptide Content, ≥80%
storage condition
protect from light
storage temp.
−20°C
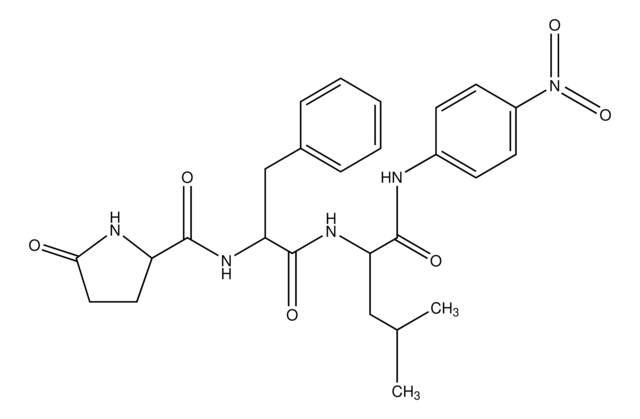
Amino Acid Sequence
Z-Arg-Arg-pNA
General description
Cathepsin B, a bilobal protein, belongs to the papain-like family of cysteine proteases. It is produced as a preproenzyme. Its catalytic site is present at the interface between the two lobes. Cathepsin B has an occluding loop. It is located in the secretory vesicles of the neuronal cells. Active cathepsin B is found in the endosomal or lysosomal compartment under normal physiological conditions.
Biochem/physiol Actions
Cathepsin B is a lysosomal cysteine proteinase that metabolizes important molecules such as β-amyloid precursor protein into harmless fragments. Cathepsin B may be detected using the chromogenic substrate Z-Arg-Arg-pNA (z-arg-arg-p-nitroanalide) or flourogenic substrate Z-Arg-Arg-AMC (z-Arg-Arg-amino-4-methylcoumarin).
Cathepsin B possesses endopeptidase and exopeptidase activity. Active cathepsin B is mostly engaged in intracellular and extracellular protein turnover, which helps cells maintain homeostatic metabolic activity. It also participates in the regulation of pro-hormone and pro-enzyme activation, antigen processing, inflammatory reactions against antigens, tissue remodeling, and apoptosis. Cathepsin B plays a key role in acute pancreatitis. It also plays a vital role in lipid metabolism in atherosclerosis.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Xiaohua Peng et al.
Journal of immunology (Baltimore, Md. : 1950), 209(7), 1314-1322 (2022-09-28)
Postviral bacterial infections are a major health care challenge in coronavirus infections, including COVID-19; however, the coronavirus-specific mechanisms of increased host susceptibility to secondary infections remain unknown. In humans, coronaviruses, including SARS-CoV-2, infect lung immune cells, including alveolar macrophages, a
S Hasnain et al.
The Journal of biological chemistry, 268(1), 235-240 (1993-01-05)
The pH dependence of cathepsin B-catalyzed hydrolyzes is very complex. At least seven dissociable groups are involved in the binding and hydrolysis of 7-amido-4-methyl coumarin and p-nitroaniline (pNA)-based substrates containing a P1 Arg and either a Phe or Arg at
Cathepsin B: Basis Sequence: Mouse.
Dora Cavallo-Medved et al.
The AFCS-nature molecule pages, 2011 (2011-01-01)
Yasuhito Sako et al.
Experimental parasitology, 127(3), 693-701 (2010-11-26)
Cysteine peptidases have potent activities in the pathogenesis of various parasitic infections, and are considered as targets for chemotherapy and antigens for vaccine. In this study, two cathepsin B-like cysteine peptidases (EmCBP1 and EmCBP2) from Echinococcus multilocularis metacestodes were identified
Ilaria Giusti et al.
Neoplasia (New York, N.Y.), 10(5), 481-488 (2008-05-14)
Vesicles shed by cancer cells are known to mediate several tumor-host interactions. Tumor microenvironment may, in turn, influence the release and the activity of tumor-shed microvesicles. In this study, we investigated the molecular mediators of the pH-dependent proinvasive activity of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service