17773
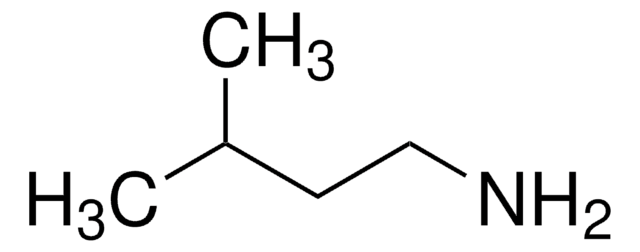
1-Amino-3-methylbutane hydrochloride
puriss., ≥98.0% (TLC)
Synonym(s):
Isopentylamine hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2CHCH2CH2NH2 · HCl
CAS Number:
Molecular Weight:
123.62
Beilstein:
3947083
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
puriss.
Quality Level
Assay
≥98.0% (TLC)
form
solid
mp
65-69 °C
SMILES string
Cl.CC(C)CCN
InChI
1S/C5H13N.ClH/c1-5(2)3-4-6;/h5H,3-4,6H2,1-2H3;1H
InChI key
HOMVDRDAAUYWKL-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
C A Ji et al.
Scientia Sinica. Series B, Chemical, biological, agricultural, medical & earth sciences, 28(11), 1188-1196 (1985-11-01)
Methylisoamylnitrosamine, a carcinogenic N-nitroso compound, has been formed in glucose ammonium nitrate medium containing 150 mg of isoamylamine (a primary amine) inoculated with a common fungus (Fusarium moniliforme Sheldon), to which 400 mg NaNO2 are added after incubation for 7-8
David M Ferrero et al.
ACS chemical biology, 7(7), 1184-1189 (2012-05-02)
Trace amine-associated receptors (TAARs) are vertebrate olfactory receptors. However, ligand recognition properties of TAARs remain poorly understood, as most are "orphan receptors" without known agonists. Here, we identify the first ligands for many rodent TAARs and classify these receptors into
Gavin L Sacks et al.
Analytical chemistry, 75(20), 5495-5503 (2004-01-09)
We report an automated method for high-precision position-specific isotope analysis (PSIA) of carbon in amino acid analogues. Carbon isotope ratios are measured for gas-phase pyrolysis fragments from multiple sources of 3-methylthiopropylamine (3MTP) and isoamylamine (IAA), the decarboxylated analogues of methionine
The deamination of isoamylamine by monamine oxidase in mitochondrial preparations from rat liver and heart: a comparison with phenylethylamine.
E M Peers et al.
Biochemical pharmacology, 29(8), 1097-1102 (1980-04-15)
C Ji et al.
Carcinogenesis, 7(2), 301-303 (1986-02-01)
Nitrosomethylisoamylamine (NMIA), a carcinogenic N-nitroso compound was synthesized from isoamylamine (IAA) in a glucose-ammonium nitrate medium after several days' incubation with fungi and subsequent nitrosation with sodium nitrate. The nitrosamine was not produced by control reactions which lacked either IAA
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service