01885
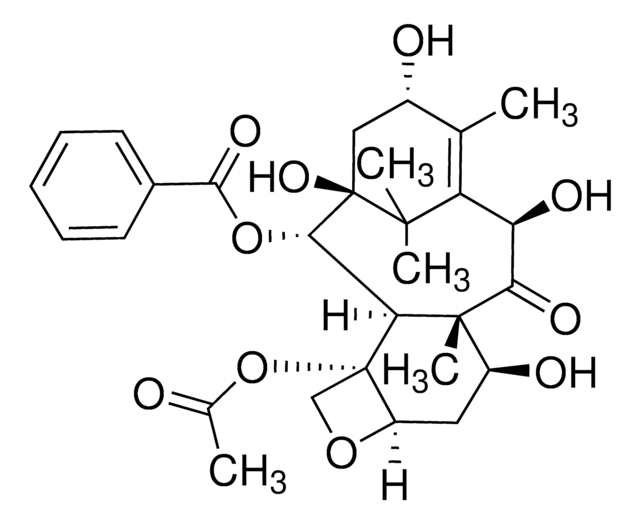
Docetaxel
purum, ≥97.0% (HPLC)
Synonym(s):
Docetaxel anhydrous
About This Item
Recommended Products
grade
purum
Quality Level
Assay
≥97.0% (HPLC)
form
powder or crystals
impurities
~6% water
mp
186-192 °C (dec.)
application(s)
metabolomics
vitamins, nutraceuticals, and natural products
SMILES string
[H][C@@]12C[C@H](O)[C@@]3(C)C(=O)[C@H](O)C4=C(C)[C@H](C[C@@](O)([C@@H](OC(=O)c5ccccc5)[C@]3([H])[C@@]1(CO2)OC(C)=O)C4(C)C)OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)c6ccccc6
InChI
1S/C43H53NO14/c1-22-26(55-37(51)32(48)30(24-15-11-9-12-16-24)44-38(52)58-39(3,4)5)20-43(53)35(56-36(50)25-17-13-10-14-18-25)33-41(8,34(49)31(47)29(22)40(43,6)7)27(46)19-28-42(33,21-54-28)57-23(2)45/h9-18,26-28,30-33,35,46-48,53H,19-21H2,1-8H3,(H,44,52)/t26-,27-,28+,30-,31+,32+,33-,35-,41+,42-,43+/m0/s1
InChI key
ZDZOTLJHXYCWBA-VCVYQWHSSA-N
Gene Information
human ... TUBA1A(7846) , TUBA1B(10376) , TUBA1C(84790) , TUBA3C(7278) , TUBA3E(112714) , TUBA4A(7277) , TUBB(203068) , TUBB1(81027) , TUBB2A(7280) , TUBB2B(347733) , TUBB3(10381) , TUBB4A(10382) , TUBB4B(10383) , TUBB6(84617) , TUBB8(347688)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- to test its effect on lysosomal autophagic flux in gastric cancer cells
- in combination with stearidonic acid (SDA) to increase its efficacy and enhancing cell death in human prostate cancer cells
- as a microtubule-stabilizing agent in prostate and cervical cancer cells
- in combination with a γ-secretase inhibitor (GSI) in prostate cancer stem-like cells (PCSCs) to test its effect on the notch signaling pathway
Biochem/physiol Actions
Packaging
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Lact. - Muta. 2 - Repr. 1B - Skin Irrit. 2 - STOT RE 1
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service