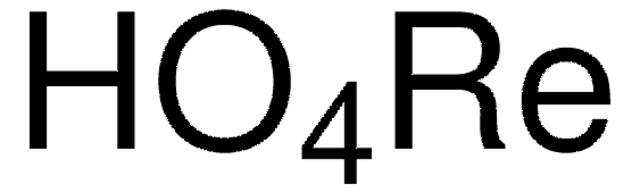All Photos(2)
About This Item
Linear Formula:
KReO4
CAS Number:
Molecular Weight:
289.30
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
99.98% trace metals basis
form
powder
impurities
≤250.0 ppm Trace Metal Analysis
mp
550 °C (lit.)
density
4.887 g/mL at 25 °C (lit.)
SMILES string
[K+].[O-][Re](=O)(=O)=O
InChI
1S/K.4O.Re/q+1;;;;-1;
InChI key
QFKRWIFGDGKWLM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
The reduction of KReO4 in the preparation of cyclopentadienyltricarbonylrhenium complexes has potential as a model for the syntheses of technetium and rhenium organometallic radiopharmaceuticals.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Spradau, T.W. Katzenellenbogen, J.A.
Organometallics, 17, 2009-2009 (1998)
Michael J Booth et al.
Science (New York, N.Y.), 336(6083), 934-937 (2012-04-28)
5-Methylcytosine can be converted to 5-hydroxymethylcytosine (5hmC) in mammalian DNA by the ten-eleven translocation (TET) enzymes. We introduce oxidative bisulfite sequencing (oxBS-Seq), the first method for quantitative mapping of 5hmC in genomic DNA at single-nucleotide resolution. Selective chemical oxidation of
Mark J Bailey et al.
Advanced healthcare materials, 1(4), 437-442 (2012-11-28)
Configuring RE(2) O(3) nanocrystals with pseudo-2D morphologies confers substantive gains in relaxivities over equivalent spherical counterparts. The most promising arises from Gd(2) O(3) whose ionic transverse and longitudinal relaxivities were as high as r(1) = 12.7 mM(-1) s(-1) and r(2)
Albert Schröckeneder et al.
Inorganic chemistry, 48(24), 11608-11614 (2009-11-27)
Rhenium(V) oxo complexes of the type [ReOX(L)(2)] (1-7; X = Cl, Br) containing beta-ketiminate ligands (L = CH(3)C(O)CH(2)C(NAr)CH(3): Ar = Ph (APOH), 2-MePh (MPOH), 2,6-Me(2)Ph (DPOH), 2,6-(i)Pr(2)Ph (DiPOH)) have been prepared by reaction of [ReOX(3)(OPPh(3))(SMe(2))] (X = Cl, Br) with
Cameron A Lippert et al.
Journal of the American Chemical Society, 132(11), 3879-3892 (2010-03-03)
Five-coordinate oxorhenium(V) anions with redox-active catecholate and amidophenolate ligands are shown to effect clean bimetallic cleavage of O(2) to give dioxorhenium(VII) products. A structural homologue with redox-inert oxalate ligands does not react with O(2). Redox-active ligands lower the kinetic barrier
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service