All Photos(1)
About This Item
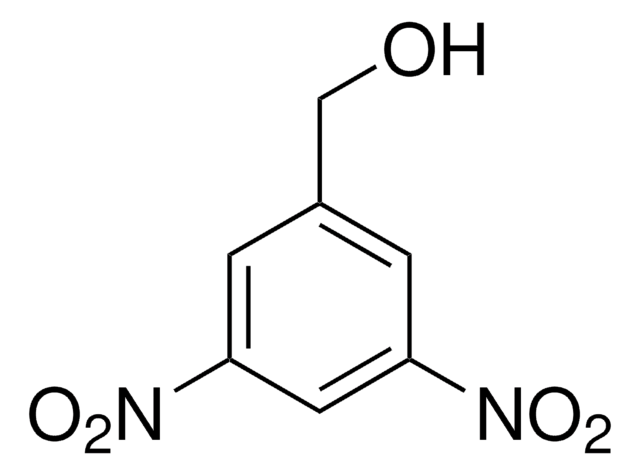
Linear Formula:
O2NC6H4CH2OH
CAS Number:
Molecular Weight:
153.14
Beilstein:
2044769
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
bp
175-180 °C/3 mmHg (lit.)
mp
26-32 °C (lit.)
density
1.29 g/mL at 20 °C (lit.)
functional group
hydroxyl
nitro
SMILES string
OCc1cccc(c1)[N+]([O-])=O
InChI
1S/C7H7NO3/c9-5-6-2-1-3-7(4-6)8(10)11/h1-4,9H,5H2
InChI key
CWNPOQFCIIFQDM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
3-Nitrobenzyl alcohol is the best substrate for cytosolic alcohol dehydrogenase.
Application
3-Nitrobenzyl alcohol was employed as matrix during fast-atom bombardment mass spectrometry. It was used in isolation of palmitylated peptide fragment from bovine rhodopsin and its characterization by mass spectrometry.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
233.6 °F - closed cup
Flash Point(C)
112 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Charlotte Mallet et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 21(21), 7944-7953 (2015-04-14)
A series of 2,5-distyrylfuran derivatives bearing pentafluorophenyl- and cyanovinyl units have been synthesized for aggregation-induced emission (AIE). The effect of the type and extent of the supramolecular connections on the AIE of the furan derivatives were examined and correlated with
S Hartmann et al.
Biological mass spectrometry, 23(6), 362-368 (1994-06-01)
Fast atom bombardment mass spectra were successfully recorded for intact glycosylphenolphthiocerol dimycocerosates (phenolic glycolipids, PGLs) from Mycobacterium kansasii, M. leprae, M. tuberculosis, M. marinum, M. bovis and M. haemophilum. Characteristic fragment ions from the loss of the oligosaccharide moiety and
Daniel D MacDougall et al.
Journal of molecular biology, 427(9), 1801-1818 (2014-10-14)
Ribosomal subunit joining is a key checkpoint in the bacterial translation initiation pathway during which initiation factors (IFs) regulate association of the 30S initiation complex (IC) with the 50S subunit to control formation of a 70S IC that can enter
Harry J Sterling et al.
Journal of the American Society for Mass Spectrometry, 23(2), 191-200 (2011-12-14)
The effects of aqueous solution supercharging on the solution- and gas-phase structures of two protein complexes were investigated using traveling-wave ion mobility-mass spectrometry (TWIMS-MS). Low initial concentrations of m-nitrobenzyl alcohol (m-NBA) in the electrospray ionization (ESI) solution can effectively increase
Harry J Sterling et al.
Physical chemistry chemical physics : PCCP, 13(41), 18288-18296 (2011-03-15)
Effects of covalent intramolecular bonds, either native disulfide bridges or chemical crosslinks, on ESI supercharging of proteins from aqueous solutions were investigated. Chemically modifying cytochrome c with up to seven crosslinks or ubiquitin with up to two crosslinks did not
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service