489477
LSD1 Inhibitor II, S2101
The LSD1 Inhibitor II, S2101 controls the biological activity of LSD1. This small molecule/inhibitor is primarily used for Cell Signaling applications.
Synonym(s):
LSD1 Inhibitor II, S2101, S2101, LSD Inhibitor II, BHC110 Inhibitor II, Histone Lysine Demethylase Inhibitor IV, KDM1 Inhibitor II, MOA Inhibitor II
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C16H16ClF2NO
Molecular Weight:
311.75
UNSPSC Code:
12352200
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥95% (HPLC)
form
solid
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
color
white
solubility
DMSO: 100 mg/mL
water: 25 mg/mL
shipped in
ambient
storage temp.
2-8°C
General description
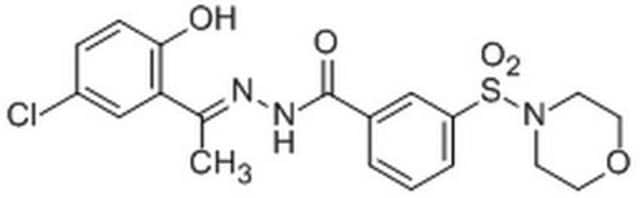
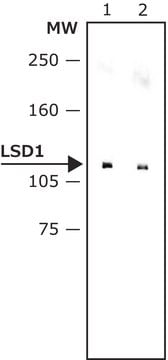
A cell-permeable, 2-PCPA derivative that elicits mechanism based inhibition of LSD1 (IC50 = 0.99 µM, KI = 0.61 µM, and kinact/KI = 4560 (M-1S-1)), and displays much lower inhibition activity toward MAO-B (KI = 17 µM, kinact/KI = 18 (M-1S-1)) and MAO-A inhibition (KI = 110 µM, kinact/KI = 60 (M-1S-1)), with higher potency and selectivity compared with 2-PCPA, IC50 = 184 µM, KI = 100 µM, kinact/KI = 58 (M-1S-1)) for LSD1, and K I = 26 µM, kinact/KI = 271 (M-1S-1)) for MAO-B, and K I = 5 µM, kinact/KI = 1050 (M-1S-1)) for MAO-A). The inhibition of LSD1 is further confirmed in a cell based assay, the treatment of HEK293T human cells with S2101 results in a dose-dependent increase in the level of H3K4me2, and about 50-fold stronger inhibition compared with that of 2-PCPA.
A cell-permeable, 2-PCPA derivative that elicits mechanism-based inhibition against LSD1 (IC50 = 0.99 µM, KI = 0.61 µM, and kinact/KI = 4560 M-1S-1), and displays much lower inhibition activity toward MAO-B (KI = 17 µM, kinact/KI = 18 M-1S-1) and MAO-A (KI = 110 µM, kinact/KI = 60 M-1S-1), with higher potency and selectivity compared with those of 2-PCPA (Cat. No. 616431) (IC50 = 184 µM, KI = 100 µM, kinact/KI = 58 M-1S-1 for LSD1, and K I = 26 µM, kinact/KI = 271 M-1S-1 for MAO-B, and K I = 5 µM,
kinact/KI = 1050 M-1S-1 for MAO-A). The inhibition of LSD1 is further confirmed in a cell-based assay, the treatment of HEK293T human cells with S2101 results in a dose-dependent increase in the level of H3K4me2, and about 50-fold stronger inhibition compared with that of 2-PCPA.
kinact/KI = 1050 M-1S-1 for MAO-A). The inhibition of LSD1 is further confirmed in a cell-based assay, the treatment of HEK293T human cells with S2101 results in a dose-dependent increase in the level of H3K4me2, and about 50-fold stronger inhibition compared with that of 2-PCPA.
Warning
Toxicity: Standard Handling (A)
Reconstitution
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 3 months at -20°C.
Other Notes
Mimasu, S., et al. 2010. Biochemistry49, 6494.
Legal Information
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Sarah A Gilmore et al.
PloS one, 17(12), e0271145-e0271145 (2022-12-09)
Chronic hepatitis B (CHB) is a global health care challenge and a major cause of liver disease. To find new therapeutic avenues with a potential to functionally cure chronic Hepatitis B virus (HBV) infection, we performed a focused screen of
Zhiqiang Huang et al.
Nucleic acids research, 51(3), 1067-1086 (2023-01-08)
The Th2 cytokine interleukin 4 (IL4) promotes macrophage differentiation into alternative subtypes and plays important roles in physiology, in metabolic and inflammatory diseases, in cancer and in tissue regeneration. While the regulatory transcription factor networks governing IL4 signaling are already
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service