549053
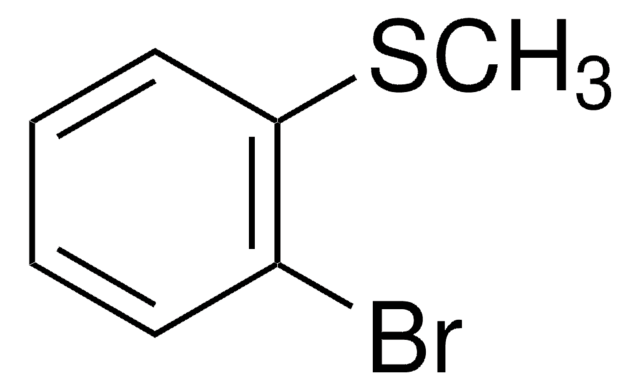
3-Bromothioanisole
97%
Synonym(s):
2-Bromophenyl methyl sulfide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
BrC6H4SCH3
CAS Number:
Molecular Weight:
203.10
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.628 (lit.)
bp
124-125 °C/10 mmHg (lit.)
density
1.51 g/mL at 25 °C (lit.)
SMILES string
CSc1cccc(Br)c1
InChI
1S/C7H7BrS/c1-9-7-4-2-3-6(8)5-7/h2-5H,1H3
InChI key
NKYFJZAKUPSUSH-UHFFFAOYSA-N
Related Categories
General description
3-Bromothioanisole is a 3-halothioanisole derivative that is mainly used in the preparation of photoacids. It can be prepared from 3-bromobenzenethiol.
Application
3-Bromothioanisole may be used in the preparation of:
- 3-methylthiotriphenylamine
- 4-ethoxy-3′-methylthiostilbene
- 3-bromophenyl phenyl sulfide
- 9-substituted, 3,6-dithiomethylfluorenes
- 4-methoxy-3′-(methylthio)-1,1′-biphenyl
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Efficient photoacids based upon triarylamine dialkylsulfonium salts.
Zhou W, et al.
Journal of the American Chemical Society, 124(9), 1897-1901 (2002)
Enhancement of acid photogeneration through a para-to-meta substitution strategy in a sulfonium-based alkoxystilbene designed for two-photon polymerization.
Xia R, et al.
Chemistry of Materials, 24(2), 237-244 (2012)
Evaluating atomic components in fluorene wires.
Klausen RS, et al.
Chemical Science, 5(4), 1561-1564 (2014)
A Preparatively Convenient Ligand-Free Catalytic PEG 2000 Suzuki- Miyaura Coupling.
Razler TM, et al.
The Journal of Organic Chemistry, 74(3), 1381-1384 (2008)
Transition-Metal-Free Acid-Mediated Synthesis of Aryl Sulfides from Thiols and Thioethers.
Wagner AM and Sanford MS.
The Journal of Organic Chemistry, 79(5), 2263-2267 (2014)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service