All Photos(2)
About This Item
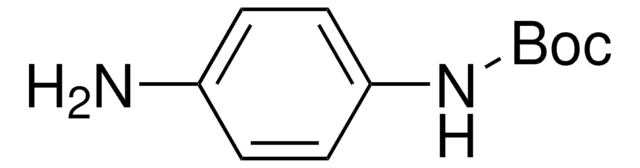
Linear Formula:
H2NC6H4CH2NHCO2C(CH3)3
CAS Number:
Molecular Weight:
222.28
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
75-78 °C (lit.)
SMILES string
CC(C)(C)OC(=O)NCc1ccc(N)cc1
InChI
1S/C12H18N2O2/c1-12(2,3)16-11(15)14-8-9-4-6-10(13)7-5-9/h4-7H,8,13H2,1-3H3,(H,14,15)
InChI key
UXWQXBSQQHAGMG-UHFFFAOYSA-N
General description
4-[(N-Boc)aminomethyl]aniline is a protected amine.
Application
4-[(N-Boc)aminomethyl]aniline may be used in the synthesis of haptens and 4-(N-boc-aminomethyl)benzene diazonium tetrafluoroborate salt.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Covalent modification of carbon nanotubes with anthraquinone by electrochemical grafting and solid phase synthesis.
Ghanem MA, et al.
Electrochimica Acta, 68, 74-80 (2012)
Sven W Meckelmann et al.
Prostaglandins & other lipid mediators, 130, 8-15 (2017-02-22)
The performance of two derivatization and ionization techniques for the quantitative reversed phase liquid chromatography (LC)- mass spectrometry (MS) analysis of hydroxy fatty acids (OH-PUFA) in plasma was evaluated: One used AMPP (N-(4-aminomethylphenyl)pyridinium chloride) leading to a positive charged amid-derivate
Staudinger ligation: a new immobilization strategy for the preparation of small-molecule arrays.
Maja Köhn et al.
Angewandte Chemie (International ed. in English), 42(47), 5830-5834 (2003-12-16)
László Szabó et al.
Materials (Basel, Switzerland), 12(1) (2019-01-10)
While intensive efforts are made to prepare carbon fiber reinforced plastics from renewable sources, less emphasis is directed towards elaborating green approaches for carbon fiber surface modification to improve the interfacial adhesion in these composites. In this study, we covalently
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service