All Photos(1)
About This Item
Empirical Formula (Hill Notation):
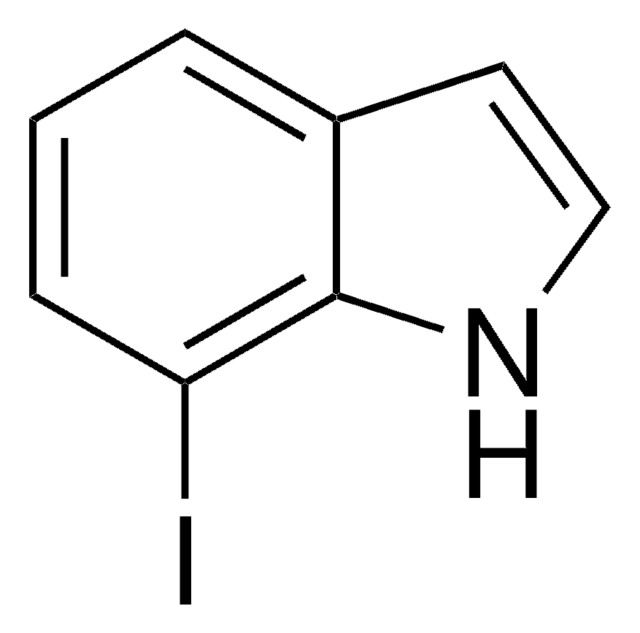
C8H6BrN
CAS Number:
Molecular Weight:
196.04
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
refractive index
n20/D 1.655 (lit.)
bp
283-285 °C (lit.)
density
1.563 g/mL at 25 °C (lit.)
SMILES string
Brc1cccc2[nH]ccc12
InChI
1S/C8H6BrN/c9-7-2-1-3-8-6(7)4-5-10-8/h1-5,10H
InChI key
GRJZJFUBQYULKL-UHFFFAOYSA-N
Application
4-Bromoindole may be used to synthesize:
- clavicipitic acid, an ergot alkaloid
- 4-bromodehydrotryptophan
- 3-indolylacetonitrile derivative
- marine alkaloid dictyodendrin B
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Andrew K Pitts et al.
Angewandte Chemie (International ed. in English), 54(18), 5451-5455 (2015-02-24)
A sequential CH functionalization strategy for the synthesis of the marine alkaloid dictyodendrin B is reported. Our synthesis begins from commercially available 4-bromoindole and involves six direct functionalizations around the heteroarene core as part of a gram-scale strategy towards the natural
S Liras et al.
Journal of the American Chemical Society, 123(25), 5918-5924 (2001-06-21)
Concise syntheses of the Ergot alkaloids rugulovasine A (3a), rugulovasine B (3b), and setoclavine (2) have been completed by strategies that feature inter- and intramolecular vinylogous Mannich reactions as the key steps. Thus, the first synthesis of 3a,b commenced with
Metal-halogen exchange of bromoindoles. A route to substituted indoles.
Moyer MP, et al.
The Journal of Organic Chemistry, 51(26), 5106- 5110 (1986)
Optically active total synthesis of clavicipitic acid.
Yokoyama Y, et al.
The Journal of Organic Chemistry, 60(6), 1486-1487 (1995)
Chaitany Jayprakash Raorane et al.
Biomolecules, 10(8) (2020-08-23)
Multi-drug resistant Acinetobacter baumannii is well-known for its rapid acclimatization in hospital environments. The ability of the bacterium to endure desiccation and starvation on dry surfaces for up to a month results in outbreaks of health care-associated infections. Previously, indole
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service