Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
G0897
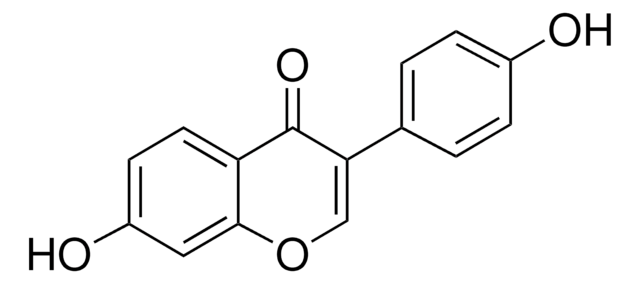
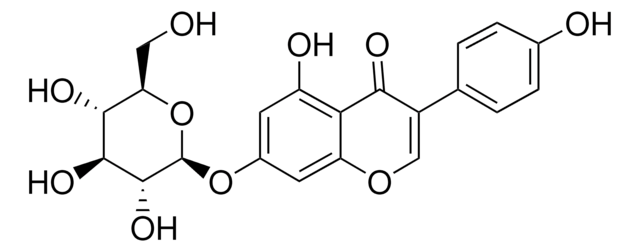
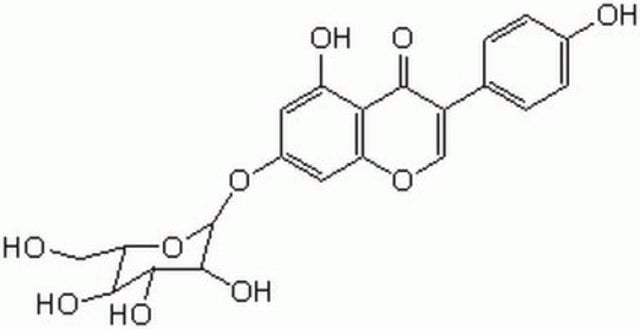
Genistin
from Glycine max (soybean), ≥95% (HPLC)
Synonym(s):
4′,5,7-Trihydroxyisoflavone 7-glucoside, Genistein 7-glucoside, Genistein-7-O-β-D-glucopyranoside, Genistoside, NSC 5112
Select a Size
About This Item
Recommended Products
biological source
Glycine max (soybean)
Quality Level
Assay
≥95% (HPLC)
solubility
DMSO: 10 mg/mL
storage temp.
−20°C
SMILES string
OC[C@H]1O[C@@H](Oc2cc(O)c3C(=O)C(=COc3c2)c4ccc(O)cc4)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C21H20O10/c22-7-15-18(26)19(27)20(28)21(31-15)30-11-5-13(24)16-14(6-11)29-8-12(17(16)25)9-1-3-10(23)4-2-9/h1-6,8,15,18-24,26-28H,7H2/t15-,18-,19+,20-,21-/m1/s1
InChI key
ZCOLJUOHXJRHDI-CMWLGVBASA-N
Looking for similar products? Visit Product Comparison Guide
Application
Biochem/physiol Actions
Features and Benefits
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Serotonin is stored in cells and metabolized by MAO, influencing CNS, GI, and platelet functions.
-
What is the Department of Transportation shipping information for this product?
1 answer-
Helpful?
-
-
What is the lambda max for genistin and what is the molar extinction coefficient at that wavelength?
1 answer-
We have data from our QC labs here at Sigma on one of the Genistin products we offer, G0897. The solvent is 85% ethanol/15% water, and the lambda max is typically 262.0 or 262.5 nm. We measured the molar extinction coefficients at the lambda max for four different lots of G0897 back in the 1990s, and the average value was 39,800 M-1.Genistin is a derivative of Genistein. There is an entry for Genistein in the chemicals encyclopedia published by the Royal Society of Chemistry, with information for Genistin under that heading.According to the chemicals encyclopedia published by the Royal Society of Chemistry, 14th edition, entry #4391, uv max (85% ethanol) 262.5 nm (a 90.5)".The value of ""a 90.5"" is the same as an E0.1%, or the extinction coefficient for a 1 mg/mL (or 1 gram per Liter) solution.If one multiplies 90.5 (L/g) by the molecular weight of genistin (432.38 g/mole), you get 39,130 M-1. This is pretty close to the value of 39,800 from our data above."
Helpful?
-
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service