The exact value will be listed on the lot specific Certificate of Analysis. A Certificate can be accessed in the DOCUMENTATION section under 'Certificate of Analysis'. Please see the link below to access a specific lot or sample Certificate:
https://www.sigmaaldrich.com/product/sigma/a1153#product-documentation
A1153
Aprotinin
3-8 TIU/mg solid, lyophilized powder
Synonym(s):
BPTI, Bovine pancreatic trypsin inhibitor, Trasylol, Trypsin inhibitor (basic)
Select a Size
Select a Size
About This Item
Recommended Products
Product Name
Aprotinin from bovine lung, lyophilized powder, 3-8 TIU/mg solid
biological source
bovine lung
Quality Level
form
lyophilized powder
specific activity
3-8 TIU/mg solid
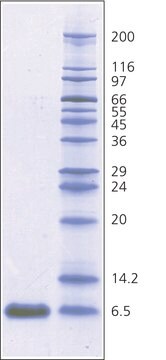
mol wt
~6,500
solubility
H2O: ≥5 mg/mL
UniProt accession no.
storage temp.
2-8°C
SMILES string
S1SCC2NC(=O)CNC(=O)CNC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C5NC(=O)C(NC(=O)CNC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C9N(CCC9)C(=O)CNC(=O)C(NC(=O)C(NC(=O)C%11N(CCC%11)C(=O
InChI
1S/C284H432N84O79S7/c1-21-144(9)222-271(439)337-174(68-46-105-309-282(300)301)239(407)340-187(120-160-77-85-164(374)86-78-160)251(419)341-185(116-156-55-29-24-30-56-156)250(418)342-188(121-161-79-87-165(375)88-80-161)252(420)346-191(123-208(291)378)246(41
InChI key
ZPNFWUPYTFPOJU-UHFFFAOYSA-N
Gene Information
cow ... PTI(404172)
Looking for similar products? Visit Product Comparison Guide
General description
Application
- as a protease inhibitor in radioimmunoprecipitation assay buffer (RIPA) for the homogenization of cardiac microvascular endothelial cells (CMECs)(4) and mammary epithelial cells[4]
- in angiogenesis assay for fibroblast[5]
- in the proteomic stabilization of saliva supernatant[6]
Biochem/physiol Actions
Unit Definition
Preparation Note
also commonly purchased with this product
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
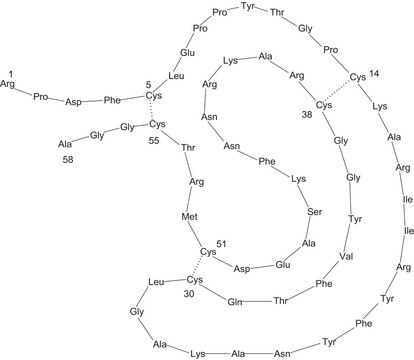
While aprotinin and bovine pancreatic trypsin inhibitor (BPTI) are the same protein sequence, the term aprotinin is typically used when describing the protein derived from bovine lung.
Elastase application index for understanding leukocyte elastase, a 29KDa serine endoprotease.
ReadyShield® phosphatase and protease inhibitor cocktail FAQ for sample protection in a variety of cell types and tissue extracts, including mammalian, plant, and microbial samples. Our ReadyShield® Protease Inhibitor Cocktail is a non-freezing solution that contains inhibitors with a broad specificity for serine, cysteine, acid proteases and aminopeptidases.
Analytical Enzyme Chymotrypsin: Chymotrypsin is produced in the acinar cells of the pancreas as the inactive precursor, chymotrypsinogen.
Protocols
Objective: To standardize a procedure for the enzymatic assay of Aprotinin.
-
What is the exact TIU/mg for the product? The information gives a range (3-8TIU/mg) but I need the exact value in order to calculate what size I need to order based on my samples.
1 answer-
Helpful?
-
-
What type of inhibitor is Aprotinin from bovine lung, Product A1153?
1 answer-
Aprotinin is a competitive serine protease inhibitor which forms stable complexes that block the active site of the enzyme.
Helpful?
-
-
How should I store solutions of Aprotinin from bovine lung, Product A1153?
1 answer-
Sterile solutions of aprotinin may be stored at 4 °C or as frozen aliquots at -20 °C.
Helpful?
-
-
When using Product A1153, Aprotinin from bovine lung, what is the conversion from KIU to TIU?
1 answer-
According to data from our labs here at Sigma, there are approximately 1300 KIU per 1 TIU. This, along with other useful information, can be found on the product information sheet available at our website: A1153 Product Information Sheet
Helpful?
-
-
Is Aprotinin from bovine lung, Product A1153, the same as Trasylol?
1 answer-
Trasylol is the Bayer AG registered trademark name for this material.
Helpful?
-
-
What are typical concentrations of Aprotinin from bovine lung, Product A1153, to use as a protease inhibitor?
1 answer-
Typical concentration of Aprotinin to be used as a protease inhibitor is 1 μM or approximately 6.5 μg/ml.
Helpful?
-
-
What is the Department of Transportation shipping information for this product?
1 answer-
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
Helpful?
-
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service