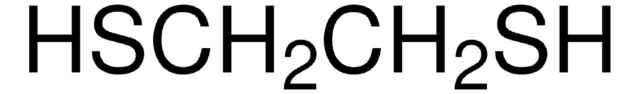77087
ω-Transaminase, Aspergillus fumigatus
recombinant, expressed in E. coli, ≥0.20 U/mg
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
CAS Number:
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
recombinant
expressed in E. coli
form
powder
specific activity
≥0.20 U/mg
storage temp.
−20°C
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Unit Definition
1 U corresponds to the amount of enzyme which releases 1 μmol acetophenone per minute at 30°C. (R(−)-α-methyl-benzylamine as substrate).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Enzymatic studies on the metabolism of beta-alanine.
O HAYAISHI et al.
The Journal of biological chemistry, 236, 781-790 (1961-03-01)
Lack of stringent stereospecificity in the inactivation of pyridoxal phosphate-dependent enzymes by suicide-substrates.
C Danzin et al.
Progress in clinical and biological research, 144A, 377-385 (1984-01-01)
Properties of the bound coenzyme and subunit structure of omega-amino acid:pyruvate aminotransferase.
K Yonaha et al.
The Journal of biological chemistry, 258(4), 2260-2265 (1983-02-25)
Hyungdon Yun et al.
Applied and environmental microbiology, 70(4), 2529-2534 (2004-04-07)
Alcaligenes denitrificans Y2k-2 was obtained by selective enrichment followed by screening from soil samples, which showed omega-amino acid:pyruvate transaminase activity, to kinetically resolve aliphatic beta-amino acid, and the corresponding structural gene (aptA) was cloned. The gene was functionally expressed in
J-S Shin et al.
Applied microbiology and biotechnology, 61(5-6), 463-471 (2003-04-11)
A transaminase from Vibrio fluvialis JS17 showing activity toward chiral amines was purified to homogeneity and its enzymatic properties were characterized. The transaminase showed an apparent molecular mass of 100 kDa as determined by gel filtration chromatography and a subunit
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service