15411
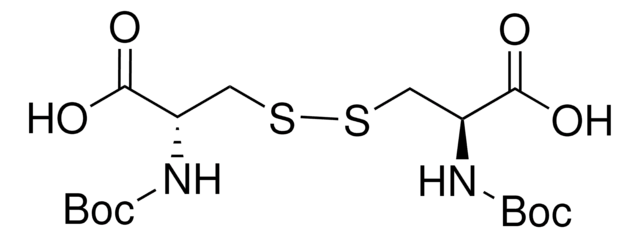
Boc-Cys-OH
for chiral derivatization, LiChropur™, ≥98.5%
Synonym(s):
Boc-L-cysteine
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C8H15NO4S
CAS Number:
Molecular Weight:
221.27
Beilstein:
2450705
MDL number:
UNSPSC Code:
41116105
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
for chiral derivatization
Quality Level
product line
ChiraSelect™
Assay
≥98.5%
form
solid
optical activity
[α]20/D +8.0±1.5°, c = 1% in ethanol
optical purity
enantiomeric ratio: ≥99.5:0.5
quality
LiChropur™
technique(s)
HPLC: suitable
SMILES string
CC(C)(C)OC(=O)N[C@@H](CS)C(O)=O
InChI
1S/C8H15NO4S/c1-8(2,3)13-7(12)9-5(4-14)6(10)11/h5,14H,4H2,1-3H3,(H,9,12)(H,10,11)/t5-/m0/s1
InChI key
ATVFTGTXIUDKIZ-YFKPBYRVSA-N
General description
Boc-L-cysteine (Boc-Cys-OH) is a high performance liquid chromatography (HPLC) grade chromatographic solvent.
Application
Boc-L-cysteine (Boc-Cys-OH) can may be used as a chromatographic solvent, in an experimental study done to study the biosynthesis of S-(3-hexan-1-ol)-glutathione (3MH-S-glut) and S-(3-hexan-l-ol)-L-cysteine (3MH-S-cys), which act as flavour precursors in wines, in Vitis vinifera grapes exposed to various environmental stress.
Other Notes
Chiral derivatizing agent used together with OPA for assaying the enantiomeric purity of amino acids
Legal Information
ChiraSelect is a trademark of Sigma-Aldrich Co. LLC
LiChropur is a trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A Hashimoto et al.
Journal of chromatography, 582(1-2), 41-48 (1992-11-06)
The concurrent determination of free amino acid enantiomers and non-chiral amino acids in rat brain and serum was accomplished by high-performance liquid chromatography with fluorimetric detection after derivatization with N-tert.-butyloxycarbonyl-L-cysteine and o-phthaldialdehyde. The method revealed the presence of a large
Reduction-responsive polymeric micelles for anticancer drug delivery.
Xulin Jiang et al.
Journal of controlled release : official journal of the Controlled Release Society, 152 Suppl 1, e36-e37 (2011-12-27)
Kouji Uda et al.
Amino acids, 48(2), 387-402 (2015-09-10)
Free D-amino acids have been found in various invertebrate phyla, while amino acid racemase genes have been identified in few species. The purpose of this study is to elucidate the distribution, function, and evolution of amino acid racemases in invertebrate
Ryushi Kawakami et al.
Journal of bioscience and bioengineering, 124(1), 23-27 (2017-03-28)
A novel amino acid racemase with broad substrate specificity (BAR) was recently isolated from the hyperthermophilic archaeon Pyrococcus horikoshii OT-3. Characterization of this enzyme has been difficult, however, because the recombinant enzyme is produced mainly as an inclusion body in
Gantumur Battogtokh et al.
Nanomedicine : nanotechnology, biology, and medicine, 18, 315-325 (2018-11-06)
Photodynamic therapy is a clinically approved treatment approach for cancer. However, it has limited applications owing to poor water solubility and the short wavelength absorption of the photosensitizer (PS). We selected a near-infrared photosensitizer, SiNC, and encapsulated into a gold
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service