526526
PIM3 Kinase Inhibitor VII, M-110
Synonym(s):
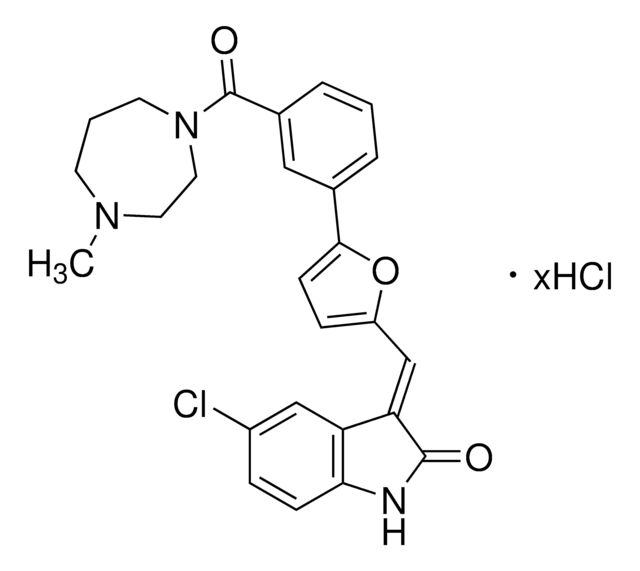
PIM3 Kinase Inhibitor VII, M-110, Nʹ-(1-(4-Chloro-2-hydroxyphenyl)propylidene)-2-((3-morpholinopropyl)amino)isonicotinohydrazide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C22H28ClN5O3
Molecular Weight:
445.94
UNSPSC Code:
12352200
NACRES:
NA.28
Recommended Products
Assay
≥97% (HPLC)
Quality Level
form
solid
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
protect from light
color
pale yellow
solubility
DMSO: 50 mg/mL
shipped in
wet ice
storage temp.
−20°C
General description
A cell-permeable hydroxyphenyl-propylidene-benzohydrazide compound that acts as a potent, ATP-competitive (Ki = 0.3 µM), and highly isoform-selective PIM inhibitor (IC50 = 2.5, 2.5, and 0.047 µM against PIM1, PIM2, and PIM3, respectively, with PIMtide as substrate, [ATP] = 10 µM), while affecting CK2α2 only at much higher concentrations (IC50 = 5 µM, [ATP] = 10 µM) and exhibiting little or no activity against a panel of 258 other kinases (<40% inhibition; [M-110] = 5 µM). M-100 treatment (10 µM for 18 hr) is shown to inhibit both basal (by 73% and 83% of DMSO control in DU-145 prostate cancer and MiaPaCa2 pancreatic cancer cells, respectively) and IL-6-stimulated (by 69% of DMSO control in DU-145 cells) STAT3 phosphorylation on Tyr705, while displaying little effect toward Tyr694 phosphoylation (in 22RV1 cultures). Isoform-specific knockdowns in DU-145 cells likewise identify PIM-3, but not PIM-1 or -2, as the kinase responsible for cellular STAT3 Tyr705 phosphorylation.
A cell-permeable hydroxyphenyl-propylidene-benzohydrazide compound that acts as a potent, ATP-competitive (Ki = 0.3 µM), and highly isoform-selective PIM inhibitor (IC50 = 2.5, 2.5, and 0.047 µM against PIM1, PIM2, and PIM3, respectively; [ATP] = 10 µM), while affecting CK2α2 only at much higher concentrations (IC50 = 5 µM, [ATP] = 10 µM) and exhibiting little or no activity against a panel of 258 other kinases (<40% inhibition at 5 µM). Shown to inhibit PIM3-dependent STAT3 Tyr705 phosphorylation in DU-145 prostate cancer and MiaPaCa2 pancreatic cancer cells (by 73% and 83%, respectively; 10 µM for 18 h), while displaying little effect toward STAT3 Tyr694 phosphoylation in 22RV1 cultures.
Packaging
Packaged under inert gas
Warning
Toxicity: Standard Handling (A)
Reconstitution
Following reconstitution, aliquot and freeze (-20°C. Stock solutions are stable for up to 6 months at -20°C.
Other Notes
Chang, M., et al. 2010. Mol. Cancer Ther.9, 2478.
Legal Information
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Mirco Glitscher et al.
Antiviral research, 226, 105891-105891 (2024-04-23)
Zoonoses such as ZIKV and SARS-CoV-2 pose a severe risk to global health. There is urgent need for broad antiviral strategies based on host-targets filling gaps between pathogen emergence and availability of therapeutic or preventive strategies. Significant reduction of pathogen
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service