All Photos(1)
About This Item
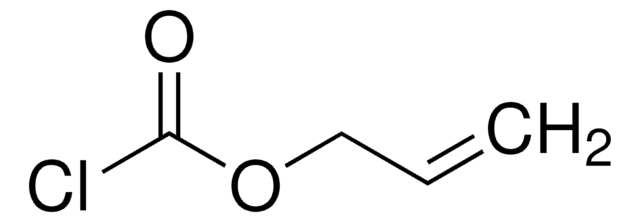
Empirical Formula (Hill Notation):
C8H9NO5
CAS Number:
Molecular Weight:
199.16
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
form
liquid
refractive index
n20/D 1.482
density
1.287 g/mL at 25 °C
storage temp.
2-8°C
SMILES string
C=CCOC(=O)ON1C(=O)CCC1=O
InChI
1S/C8H9NO5/c1-2-5-13-8(12)14-9-6(10)3-4-7(9)11/h2H,1,3-5H2
InChI key
OIXALTPBNZNFLJ-UHFFFAOYSA-N
Related Categories
Application
N-(Allyloxycarbonyloxy)succinimide (alloc-Su) can be used as:
- A building block for the preparation of glycopeptide scaffolds.
- A reagent in the synthesis of various functional cyclic carbonate monomers from 2-amino-1,3-propane diols.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
242.6 °F
Flash Point(C)
117 °C
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
2-Amino-1, 3-propane diols: a versatile platform for the synthesis of aliphatic cyclic carbonate monomers
Venkataraman S, et al.
Polym. Chem., 4(10), 2945-2948 (2013)
Bo Wu et al.
Molecules (Basel, Switzerland), 25(19) (2020-10-07)
Four bis-lactam [i, i+4]-stapled peptides with d- or l-α-methyl-thialysines were constructed on a model peptide sequence derived from p110α[E545K] and subjected to circular dichroism (CD) and proteolytic stability assessment, alongside the corresponding bis-lactam [i, i+4]-stapled peptide with l-thialysine. The %
Synthesis and in vitro evaluation of bisphosphonated glycopeptide prodrugs for the treatment of osteomyelitis
Tanaka KSE, et al.
Bioorganic & medicinal chemistry letters, 20(4), 1355-1359 (2010)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service