All Photos(1)
About This Item
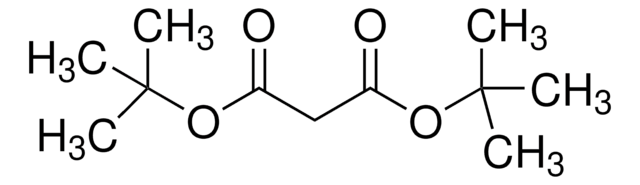
Linear Formula:
H2C=CHCH2CH(CO2CH3)2
CAS Number:
Molecular Weight:
172.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
refractive index
n20/D 1.435 (lit.)
bp
207 °C/771 mmHg (lit.)
density
1.071 g/mL at 25 °C (lit.)
SMILES string
COC(=O)C(CC=C)C(=O)OC
InChI
1S/C8H12O4/c1-4-5-6(7(9)11-2)8(10)12-3/h4,6H,1,5H2,2-3H3
InChI key
VZNFVLWVVHHMBG-UHFFFAOYSA-N
Related Categories
Application
Dimethyl allylmalonate may be used in the preparation of:
- terminal alkyl silane
- diallyl malonates
- bromosulfoxide
- dimethyl-2-allyl-2-{4-methyl-2-[(tetrahydro-2H-pyran-2-yloxy)methyl]deca-2,3-dienoyl}malonate
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
199.4 °F - closed cup
Flash Point(C)
93 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of substituted cyclopentenes from the Baylis-Hillman adducts via ring-closing metathesis reaction.
Lee KY, et al.
Bull. Korean Chem. Soc., 25(8), 1280-1282 (2004)
The study of intramolecular free radical cyclizations of a-sulfonyl and a-sulfinyl radicals.
Yeun-Min T, et al.
Tetrahedron Letters, 31(42), 6047-6050 (1990)
Tandem cyclization/hydrosilylation of functionalized 1, 6-dienes catalyzed by a cationic palladium complex.
Widenhoefer RA and DeCarli MA.
Journal of the American Chemical Society, 120(15), 3805-3806 (1998)
Krause N.
Science of Synthesis: Houben-Weyl Methods of Molecular Transformations, 138-138 (2014)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service