All Photos(1)
About This Item
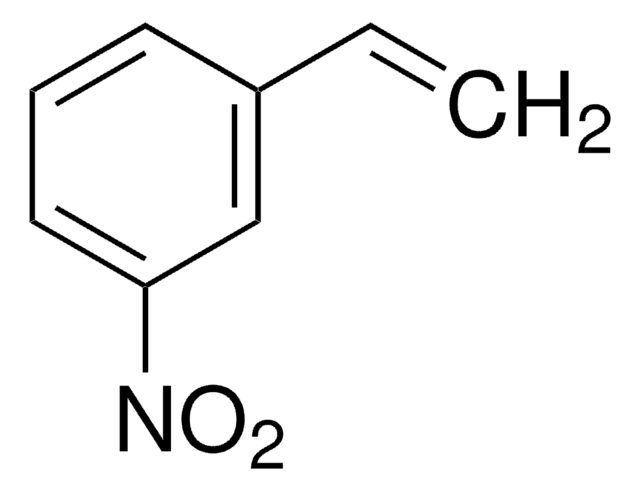
Linear Formula:
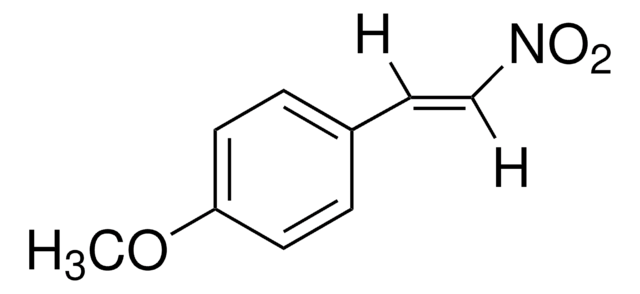
CH3C6H4CH=CHNO2
CAS Number:
Molecular Weight:
163.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
102-104 °C (lit.)
storage temp.
2-8°C
SMILES string
[H]\C(=C(\[H])[N+]([O-])=O)c1ccc(C)cc1
InChI
1S/C9H9NO2/c1-8-2-4-9(5-3-8)6-7-10(11)12/h2-7H,1H3/b7-6+
InChI key
JSPNBERPFLONRX-VOTSOKGWSA-N
Related Categories
General description
trans-4-Methyl-β-nitrostyrene ((E)-1-methyl-4-(2-nitrovinyl)benzene) is a nitrolefin. Its asymmetric Michael addition with benzaldehyde in the presence of silylated pyrrolidine catalyst has been reported. Its hydrogenation in the presence of Pd(II) complexes of (Z)-2-((quinolin-3-ylimino)methyl)phenol as catalyst has been studied.
Application
trans-4-Methyl-β-nitrostyrene may be used as a reagent in the synthesis of N-benzylpyrrolomorphinans and 4-oxo-2-aryl-4H-chromene-3-carboxylate derivatives.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Pd (II) complexes based on quinoline derivative: Structural characterization and their role as a catalyst for hydrogenation of (E)-1-methyl-4-(2-nitrovinyl) benzene.
Azam M, et al.
Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy, 123, 1-6 (2014)
Silylated pyrrolidines as catalysts for asymmetric Michael additions of aldehydes to nitroolefins.
Ralph Husmann et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 16(42), 12549-12552 (2010-09-30)
Sanjay K Srivastava et al.
Journal of medicinal chemistry, 45(2), 537-540 (2002-01-11)
A new method for the preparation of N-benzylpyrrolomorphinans has been developed. Thus Michael reaction of the benzylimines of oxycodones and oxymorphones with nitrostyrenes gave a series of 4'-aryl-N-benzylpyrrolomorphinans. These were selective delta antagonists of much higher in vitro potency (with
Manoj R Zanwar et al.
The Journal of organic chemistry, 77(15), 6495-6504 (2012-07-20)
The unusual alcohol mediated formation of 4-oxo-2-aryl-4H-chromene-3-carboxylate (flavone-3-carboxylate) derivatives from 4-hydroxycoumarins and β-nitroalkenes in an alcoholic medium is described. The transformation occurs via the in situ formation of a Michael adduct, followed by the alkoxide ion mediated rearrangement of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service