All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H7NO2
CAS Number:
Molecular Weight:
173.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
183-185 °C (lit.)
functional group
carboxylic acid
SMILES string
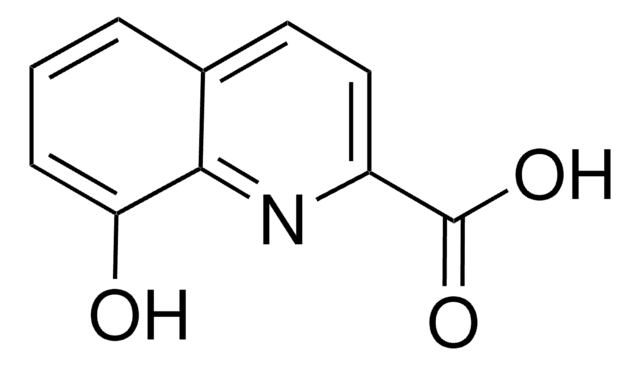
OC(=O)c1cccc2cccnc12
InChI
1S/C10H7NO2/c12-10(13)8-5-1-3-7-4-2-6-11-9(7)8/h1-6H,(H,12,13)
InChI key
QRDZFPUVLYEQTA-UHFFFAOYSA-N
General description
Herbicide 8-quinolinecarboxylic acid and its removal from aqueous solution using sodium montmorillonite, acidic montmorillonite and organo-acidic montmorillonite has been reported.
Application
8-Quinolinecarboxylic acid may be used in the synthesis of:
- novel oxorhenium(V) complexes incorporating quinoline and isoquinoline carboxylic acid derivatives
- chiral 1,2,3,4-tetrahydroquinolinyl-oxazoline compounds, used as ligands for Ru-catalyzed asymmetric transfer hydrogenation of ketones
- chiral quinolinyl-oxazoline compounds, used as ligands for Cu(II) catalyzed asymmetric cyclopropanation
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
S López-Bernabeu et al.
Chemosphere, 144, 982-988 (2015-10-05)
The effect of the electrochemical treatment (potentiostatic treatment in a filter-press electrochemical cell) on the adsorption capacity of an activated carbon cloth (ACC) was analyzed in relation with the removal of 8-quinolinecarboxylic acid pollutant from water. The adsorption capacity of
Chiral quinolinyl-oxazolines as ligands for copper (I)-catalyzed asymmetric cyclopropanation.
Wu X-Y, et al.
Tetrahedron Asymmetry, 9(23), 4143-4150 (1998)
Chiral 1, 2, 3, 4-tetrahydroquinolinyl-oxazoline ligands for Ru-catalyzed asymmetric transfer hydrogenation of ketones.
Zhou Y-B, et al.
Tetrahedron Asymmetry, 13(5), 469-473 (2002)
Barbara Machura et al.
Dalton transactions (Cambridge, England : 2003), 42(24), 8827-8837 (2013-05-04)
Six novel oxorhenium(V) complexes incorporating quinoline and isoquinoline carboxylic acid derivatives were prepared in good yields. Relying on the experimental conditions, compounds with two chelate ligands [ReOCl(iqc)2]·MeOH (1), [ReO(OMe)(iqc)2] (2), [ReO(OMe)(mqc)2] (3) and [ReO(OMe)(8-qc)2] (4) and compounds incorporating one bidentate
M Mekhloufi et al.
Environmental monitoring and assessment, 185(12), 10365-10375 (2013-08-09)
Sodium montmorillonite (Na-M), acidic montmorillonite (H-M), and organo-acidic montmorillonite (Org-H-M) were applied to remove the herbicide 8-quinolinecarboxylic acid (8-QCA). The montmorillonites containing adsorbed 8-QCA were investigated by transmission electron microscopy, FT-IR spectroscopy, X-ray diffraction analysis, X-ray fluorescence thermogravimetric analysis, and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service