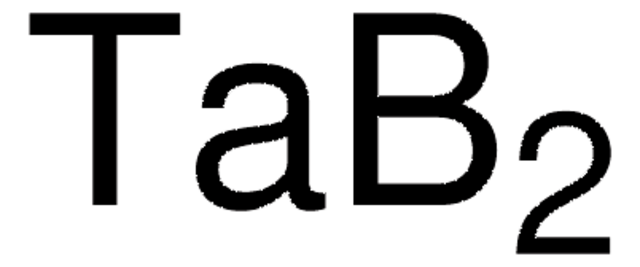All Photos(1)
About This Item
Linear Formula:
TiB2
CAS Number:
Molecular Weight:
69.49
EC Number:
MDL number:
UNSPSC Code:
12352300
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
form
powder
particle size
<10 μm
density
4.52 g/mL at 25 °C (lit.)
SMILES string
B#[Ti]#B
InChI
1S/2B.Ti
InChI key
QYEXBYZXHDUPRC-UHFFFAOYSA-N
Related Categories
Application
- Surface Modifications to Reduce Wear in Hot Extrusion of Copper: Discusses the use of titanium boride in surface treatments to enhance the wear resistance of tools used in the hot extrusion of copper (Thewes et al., 2024).
- Stable DC Vacuum Arc Plasma Generation from a 100 mm TiB2 Cathode: Explores the use of titanium boride in generating stable plasma for coatings, emphasizing its efficiency and stability in industrial applications (Zhirkov et al., 2024).
- Structure Searches and Superconductor Discovery in XB2: Investigates various titanium boride compounds in the context of their structural properties and potential superconductivity applications (Meng et al., 2024).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Marta Pegueroles et al.
Biointerphases, 7(1-4), 48-48 (2012-08-10)
An understanding of protein adsorption process is crucial for designing biomaterial surfaces. In this work, with the use of a quartz-crystal microbalance with dissipation monitoring, we researched the following: (a) the kinetics of adsorption on TiO(2) surfaces of three extensively
Pengyu Dong et al.
Nanoscale, 4(15), 4641-4649 (2012-06-22)
Graphene sheets were obtained through solvothermal reduction of colloidal dispersion of graphene oxide in benzyl alcohol. The graphene/rod-shaped TiO(2) nanocomposite was synthesized by this novel and facile solvothermal method. During the solvothermal reaction, both the reduction of graphene oxide and
G S Vinod Kumar et al.
Physical chemistry chemical physics : PCCP, 9(48), 6415-6425 (2007-12-07)
In the present paper the authors studied isolated metallic films made from the same material used for making metallic foams, and then characterised their properties. Metal films were made from a liquid aluminium alloy reinforced with ceramic particles of known
S Dohshi et al.
Journal of nanoscience and nanotechnology, 1(3), 337-342 (2003-08-14)
Characterizations of Ti-B binary oxide thin films by means of various spectroscopic measurements have shown that Ti-B binary oxide thin films are formed by ultra fine TiO2 nanoparticles. A dramatic decrease in the contact angle of water droplets to 0
Benoit Van Aken et al.
Water science and technology : a journal of the International Association on Water Pollution Research, 64(6), 1226-1232 (2012-01-05)
Extracellular DNA in municipal wastewater and effluents from hospitals and R&D laboratories contains antimicrobial resistance and recombinant genes that are today considered as a new class of emerging contaminants. The objective of this research was to investigate the effect of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service