All Photos(2)
About This Item
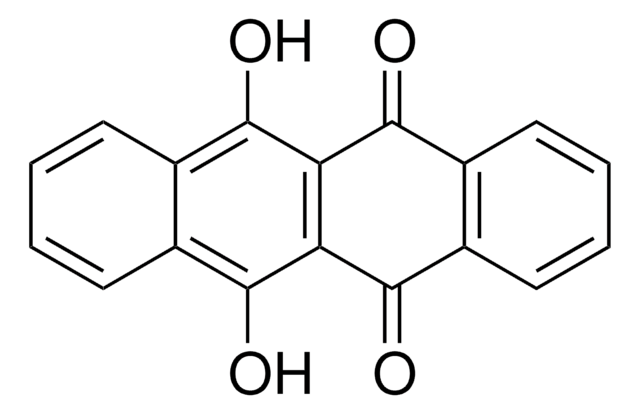
Empirical Formula (Hill Notation):
C18H10O2
CAS Number:
Molecular Weight:
258.27
Beilstein:
1880180
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
282-286 °C (dec.) (lit.)
functional group
ketone
SMILES string
O=C1c2ccccc2C(=O)c3cc4ccccc4cc13
InChI
1S/C18H10O2/c19-17-13-7-3-4-8-14(13)18(20)16-10-12-6-2-1-5-11(12)9-15(16)17/h1-10H
InChI key
LZPBKINTWROMEA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The electronic structure of the lowest excited triplet state of 5,12-naphthacenequinone was studied using pulsed electron nuclear double resonance and continuous-wave time-resolved EPR (cw-TREPR).
Application
5,12-Naphthacenequinone was used to study the phototransformation of phenol in aqueous solution.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Takuji Shimokage et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 58(6), 1201-1208 (2002-05-08)
Continuous-wave time-resolved EPR (cw-TREPR) and pulsed electron nuclear double resonance (ENDOR) studies have been carried out to clarify the electronic structure of the lowest excited triplet (Tl) state of 5,12-naphthacenequinone (5,12-NpQ) as well as 1,4-anthraquinone (1,4-AQ) and 6,13-pentacenequinone (6,13-PeQ). The
Valter Maurino et al.
Physical chemistry chemical physics : PCCP, 13(23), 11213-11221 (2011-05-17)
The phototransformation of phenol in aqueous solution was studied with different quinoid compounds, which are usually detected on atmospheric particulate matter: 2-ethylanthraquinone (EtAQ), benzanthracene-7,12-dione (BAD), 5,12-naphthacenequinone (NQ), 9,10-anthraquinone (AQ), and 2,6-dihydroxyanthraquinone (DAQ). All the studied quinones were able to sensitise
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service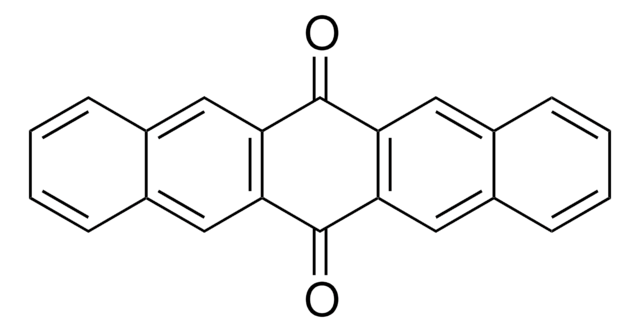




![9,11,20,22-TETRAPHENYLTETRABENZO[A,C,L,N]PENTACENE-10,21-DIONE AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/202/902/2e5e4f51-7577-47a6-8ced-ecc3534b3a79/640/2e5e4f51-7577-47a6-8ced-ecc3534b3a79.png)


