All Photos(1)
About This Item
Linear Formula:
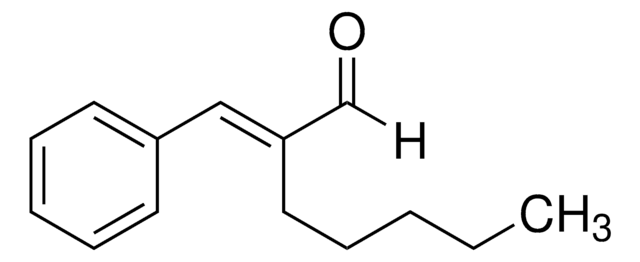
C6H5CH=C(CH3)CHO
CAS Number:
Molecular Weight:
146.19
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.605 (lit.)
bp
148-149 °C/27 mmHg (lit.)
density
1.047 g/mL at 25 °C (lit.)
SMILES string
[H]C(=O)\C(C)=C(/[H])c1ccccc1
InChI
1S/C10H10O/c1-9(8-11)7-10-5-3-2-4-6-10/h2-8H,1H3/b9-7+
InChI key
VLUMOWNVWOXZAU-VQHVLOKHSA-N
Biochem/physiol Actions
α-Methyl trans cinnamaldehyde has antifungal activity. It is self coupled and complexed with Co(II) and Ni(II) to synthesize ligand and complexes.
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
174.2 °F - closed cup
Flash Point(C)
79 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Sheikh Shreaz et al.
Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine, 24(5), 923-933 (2011-04-09)
Antifungal effectivity and utility of cinnamaldehyde is limited because of its high MIC and skin sensitivity. In this study, α-methyl trans cinnamaldehyde, a less irritating derivative, have been self coupled and complexed with Co(II) and Ni(II) to generate N, N'-Bis
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service