860602P
Avanti
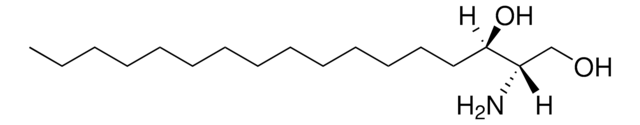
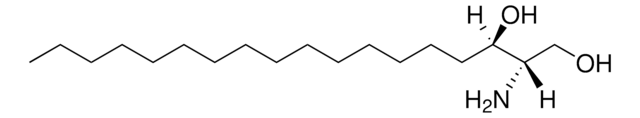
D-ribo-phytosphingosine (C17 base)
Avanti Research™ - A Croda Brand 860602P, powder
Synonym(s):
4-hydroxysphinganine (C17 base)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C17H37NO3
CAS Number:
Molecular Weight:
303.48
UNSPSC Code:
12352211
NACRES:
NA.25
Recommended Products
form
powder
packaging
pkg of 1 × 10 mg (860602P-10mg)
pkg of 1 × 5 mg (860602P-5mg)
manufacturer/tradename
Avanti Research™ - A Croda Brand 860602P
lipid type
sphingolipids
shipped in
dry ice
storage temp.
−20°C
SMILES string
O[C@]([H])(CCCCCCCCCCCCC)[C@](O)([H])[C@](N)([H])CO
General description
D-ribo-phytosphingosine (C17 base), also known as 4D-hydroxysphinganine or PHS, is widely found in membranes of fungi, plants, bacteria, marine organisms and mammalian tissues.
Application
D-ribo-phytosphingosine (C17 base) has been used as a standard for the quantification of total plant long-chain bases (LCB) by gas chromatography-mass spectrometry (GC-MS).
Biochem/physiol Actions
D-ribo-phytosphingosine helps in maintaining the structural integrity of membrane. It also controls cellular growth and mediates the heat stress response of yeast. In addition, PHS acts as a precursor for synthesis of various key lipid mediators including PHS 1-phosphate, inositol phosphorylceramide and KRN7000 (α-anomer of galactosylceramide). This phospholipid also has an ability to stimulate keratinocyte differentiation. Therefore, PHS is used as an active constituent in cosmetic formulations.
Packaging
5 mL Amber Glass Screw Cap Vial (860602P-10mg)
5 mL Amber Glass Screw Cap Vial (860602P-5mg)
Legal Information
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Polar emollients in cosmetic formulations enhance the penetration and biological effects of Phytosphingosine on skin
Schiemann Y, et al.
Colloids and Surfaces. A, Physicochemical and Engineering Aspects, 331(1-2), 103-107 (2008)
Asymmetric synthesis of d-ribo-phytosphingosine from 1-tetradecyne and (4-methoxyphenoxy) acetaldehyde
Liu Z, et al.
The Journal of Organic Chemistry, 75(13), 4356-4364 (2010)
Zheng Liu et al.
The Journal of organic chemistry, 75(13), 4356-4364 (2010-06-10)
An asymmetric synthesis of d-ribo-phytosphingosine (1) was achieved by utilizing the ProPhenol (12)-catalyzed alkynylation of unsaturated aldehyde 8 to afford allylic propargylic alcohol (S)-6 followed by asymmetric epoxidation and opening of propargylic epoxy alcohol anti-5 with NaN(3)/NH(4)Cl. Deprotection and reduction
Jean-Luc Cacas et al.
Analytical and bioanalytical chemistry, 403(9), 2745-2755 (2012-05-12)
In eukaryotic organisms, sphingolipids are major structural lipids of biological membranes and perform additional essential functions as signalling molecules. While long-chain bases (LCB), the common precursor to all sphingolipid classes, is represented by only one major molecular species in animals
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service