B4007
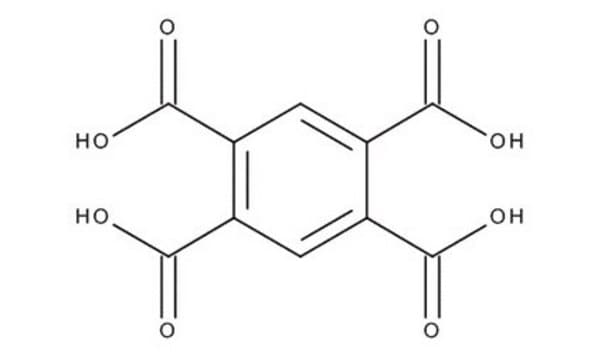
1,2,4,5-Benzenetetracarboxylic acid
96%
Synonym(s):
Pyromellitic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
C6H2(CO2H)4
CAS Number:
Molecular Weight:
254.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
form
powder
mp
281-284.5 °C (lit.)
SMILES string
OC(=O)c1cc(C(O)=O)c(cc1C(O)=O)C(O)=O
InChI
1S/C10H6O8/c11-7(12)3-1-4(8(13)14)6(10(17)18)2-5(3)9(15)16/h1-2H,(H,11,12)(H,13,14)(H,15,16)(H,17,18)
InChI key
CYIDZMCFTVVTJO-UHFFFAOYSA-N
Related Categories
Application
1,2,4,5-Benzenetetracarboxylic acid is extensively used as a ligand in the synthesis of a wide range of metal-organic frameworks (MOFs) and coordination polymers. It can also be used to prepare supramolecular hydrogels by synthesizing gelators reacting 1,2,4,5-benzenetetracarboxylic acid with 4-hydroxy pyridine.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
617.0 °F
Flash Point(C)
325 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
X Xiong et al.
Electrophoresis, 19(12), 2243-2251 (1998-10-07)
The simultaneous separation and detection of small cations and anions by capillary zone electrophoresis (CZE) with indirect ultraviolet (UV) detection was successfully demonstrated in a background electrolyte (BGE) containing two UV-absorbing components. Benzylamine, imidazole, benzenesulfonic acid, sulfosalicylic acid, and pyromellitic
Astrid Barkleit et al.
Inorganic chemistry, 50(12), 5451-5459 (2011-05-25)
Thermodynamic parameters for the complexation of Eu(3+) with pyromellitic acid (1,2,4,5-benzenetetracarboxylic acid, BTC) as a model system for polymerizable metal-complexing humic acids were determined using temperature-dependent time-resolved laser-induced fluorescence spectroscopy (TRLFS) and isothermal titration calorimetry (ITC). At low metal and
Jacek Gawroński et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 8(11), 2484-2494 (2002-08-16)
The chiral but highly symmetrical acyclic and cyclic pyromellitic diimide dimers and trimers 2-5 have been obtained and characterized for the first time. The pyromellitdiimide chromophores in these molecules are linked by a rigid diequatorially 1,2-disubstituted cyclohexane skeleton. The structures
1, 2, 4, 5?Benzenetetracarboxylic Acid and 4, 4??Bipyridine as Ligands in Designing Low?Dimensional Coordination Polymers.
Ruiz?Perez C, et al.
European Journal of Inorganic Chemistry, 2004(19), 3873-3879 (2004)
Yu Liu et al.
Carbohydrate research, 338(17), 1751-1757 (2003-08-02)
A novel bridged bis(beta-cyclodextrin) with a pyromellitic acid 2,5-diamide tether (2) has been synthesized by reaction of 6(I)-(2-aminoethyleneamino)-6-deoxycyclomaltoheptaose [mono 6-(2-aminoethyleneamino)-6-deoxy-beta-cyclodextrin] with 1,2,4,5-benzenetetracarboxylic dianhydride. Its inclusion complexation behavior with some representative dyestuffs, i.e., Acridine Red (AR), Rhodamine B (RhB), Neutral Red
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service