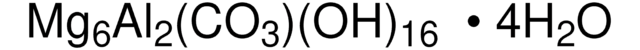69866
Montmorillonite
K 10, powder
Synonym(s):
K-Catalyst
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
form
powder
pH
3-4
InChI
1S/2Al.4O2Si.H2O.3O/c;;4*1-3-2;;;;/h;;;;;;1H2;;;/q2*+3;;;;;;3*-2
InChI key
GUJOJGAPFQRJSV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Montmorillonite is a layered clay mineral (2:1 phyllosilicate) that belongs to a class of the smectite group. It is a naturally abundant material and is chemically modified by the cation-exchange method for the preparation of polymer nanocomposites.
Application
Montmorillonite has been used as:
It can also be used:
- A nanofiller to improve the mechanical properties of poly(vinyl alcohol) (PVA) film for potential usage in packaging applications.
- A catalyst in the conversion of polar and non-polar algae oil lipids to biodiesel.
It can also be used:
- As a nanofiller in the preparation of biopolymer based nanocomposites.
- In the preparation of Fe3+-montmorillonite, a solid acid catalyst for the synthesis of xanthenedione derivatives.
Other Notes
Solid Lewis acid catalyst
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Conversion of polar and non-polar algae oil lipids to fatty acid methyl esters with solid acid catalysts-A model compound study.
Asikainen M, et al.
Bioresource Technology, 191(1-2), 300-305 (2015)
Fe3+-montmorillonite as a cost-effective and recyclable solid acidic catalyst for the synthesis of xanthenediones.
Song G, et al.
Catalysis Communications, 8(4), 673-676 (2007)
O. Sieskind et al.
Tetrahedron Letters, 34, 1197-1197 (1993)
P Laszlo
Science (New York, N.Y.), 235(4795), 1473-1477 (1987-03-20)
Layer aluminosilicates catalyze reactions in numerous ways. They stabilize high-energy intermediates. They can store energy in their lattice structures and can release it in the form of chemical energy. They can catalyze redox reactions and can serve as photocatalytic devices.
Poly (vinyl alcohol): Montmorillonite: Boiled rice water (starch) blend film reinforced with silver nanoparticles; characterization and antibacterial properties.
Mathew S, et al.
Applied Clay Science, 161(1-2), 464-473 (2018)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service