All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H6ClN5O2
CAS Number:
Molecular Weight:
227.61
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
>300 °C (lit.)
SMILES string
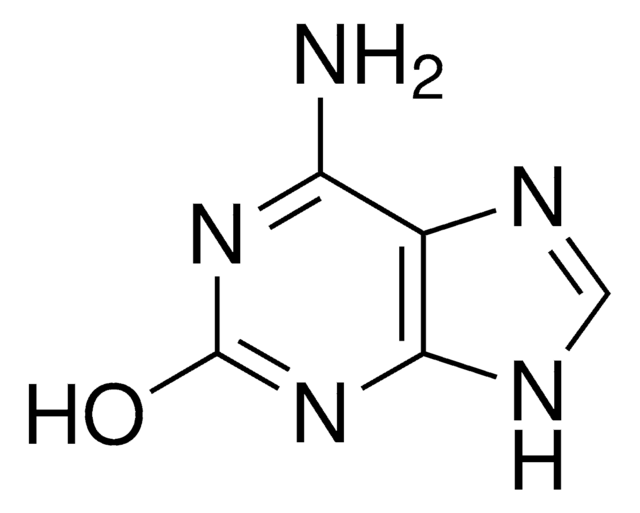
Nc1nc(Cl)c2ncn(CC(O)=O)c2n1
InChI
1S/C7H6ClN5O2/c8-5-4-6(12-7(9)11-5)13(2-10-4)1-3(14)15/h2H,1H2,(H,14,15)(H2,9,11,12)
InChI key
LRZBBTTUKFVUMZ-UHFFFAOYSA-N
General description
2-Amino-6-chloro-9H-purine-9-acetic acid is a purine derivative.
Application
2-Amino-6-chloro-9H-purine-9-acetic acid may be used as starting material in the synthesis of:
- 6-decyloxy substituted purine intermediates
- 6-decylthio substituted purine intermediates
- 6-decyloxy or 6-decylthio-9-phenylalanine/serine or alanine-substituted purine derivatives
- 2,6-diaminopurine monomer
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Damian Ackermann et al.
Nucleic acids research, 41(8), 4729-4739 (2013-02-28)
The structural reorganization of nanoscale DNA architectures is a fundamental aspect in dynamic DNA nanotechnology. Commonly, DNA nanoarchitectures are reorganized by means of toehold-expanded DNA sequences in a strand exchange process. Here we describe an unprecedented, toehold-free switching process that
Ashish K Pathak et al.
Bioorganic & medicinal chemistry, 21(7), 1685-1695 (2013-02-26)
6-Oxo and 6-thio analogs of purine were prepared based on the initial activity screening of a small, diverse purine library against Mycobacterium tuberculosis (Mtb). Certain 6-oxo and 6-thio-substituted purine analogs described herein showed moderate to good inhibitory activity. N(9)-substitution apparently
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service