All Photos(1)
About This Item
Linear Formula:
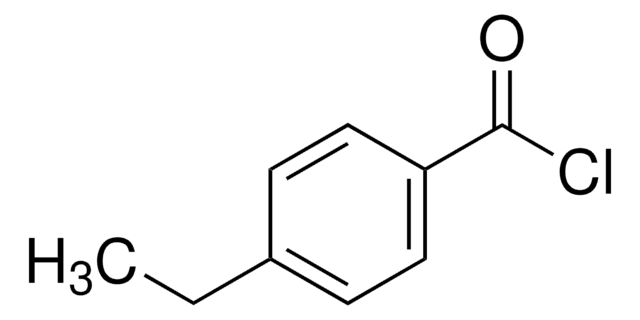
ClC6H3(NO2)COCl
CAS Number:
Molecular Weight:
220.01
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
98%
mp
47-54 °C (lit.)
functional group
acyl chloride
chloro
nitro
SMILES string
[O-][N+](=O)c1cc(ccc1Cl)C(Cl)=O
InChI
1S/C7H3Cl2NO3/c8-5-2-1-4(7(9)11)3-6(5)10(12)13/h1-3H
InChI key
IWLGXPWQZDOMSB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Chloro-3-nitrobenzoyl chloride is an acid halide.
Application
4-Chloro-3-nitrobenzoyl chloride may be employed as acylation reagent for the Friedel-Crafts acylation of activated benzenes such as anisole, veratrole and 1,4-dimethoxybenzene. It may be employed for the synthesis of 4-chloro-4-methoxy-3-nitrobenzophenone. It may be employed as acylation reagent for the acylation reaction of the deactivated amines (2- aminopyrimidine, aminopyrazine).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Insights into the N,N-diacylation reaction of 2-aminopyrimidines and deactivated anilines: an alternative N-monoacylation reaction.
Theodorou V, et al.
ARKIVOC (Gainesville, FL, United States), 4, 11-23 (2014)
Tzvetomira Tzanova et al.
European journal of medicinal chemistry, 44(6), 2724-2730 (2008-10-28)
Considering that oxidative stress is strongly implicated in the toxicity of chemotherapy, much effort is focused on the research of diverse antioxidants as protective agents. An efficient synthesis of three novel benzophenones containing 1,3-thiazol moiety (6a-c) is described. Their antioxidant
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service