All Photos(1)
About This Item
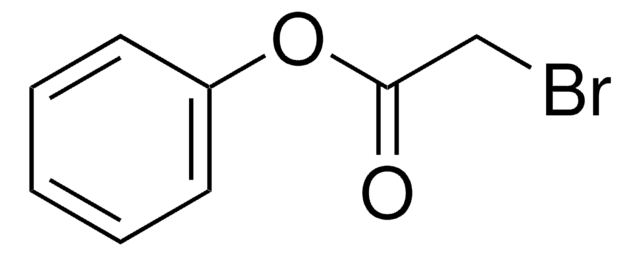
Linear Formula:
ClCH2CO2CH2C6H5
CAS Number:
Molecular Weight:
184.62
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
99%
refractive index
n20/D 1.525 (lit.)
bp
90 °C/1 mmHg (lit.)
density
1.215 g/mL at 25 °C (lit.)
functional group
chloro
ester
phenyl
SMILES string
ClCC(=O)OCc1ccccc1
InChI
1S/C9H9ClO2/c10-6-9(11)12-7-8-4-2-1-3-5-8/h1-5H,6-7H2
InChI key
SOGXBRHOWDEKQB-UHFFFAOYSA-N
General description
Benzyl chloroacetate is an ester.
Application
Benzyl chloroacetate may be used:
- in the synthesis of monoesters of phosphonoacetic acid
- in the synthesis of N-benzoyl-glycyl- hydroxyl acetic acid, ester substrate for carboxypeptidase Y catalyzed peptide synthesis
- as reagent in the total syntheses of the (±)-di-O-methyl ethers of the norlignans sequirin-A, agatharesinol, and hinokiresinol, and of (±)-tri-O-methyl sequirin-E
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Influence of the substrate structure on carboxypeptidase Y catalyzed peptide bond formation.
Breddam K, et al.
Carlsberg Research Communications, 45(5), 361-367 (1980)
T R Herrin et al.
Journal of medicinal chemistry, 20(5), 660-663 (1977-05-01)
The synthesis of monoesters (P and C) of phosphonoacetic acid (PA) is given. The carboxyl esters were prepared by two methods: the reaction of chloroacetates with tris(trimethylsilyl) phosphite, followed by hydrolysis; and by the acid-catalyzed esterification of PA with the
Stereoselective total syntheses of the (?)-di-O-methyl ethers of agatharesinol, sesquirin-A, and hinokiresinol, and of (?)-tri-O-methylsequirin-E, characteristic norlignans of coniferae.
Beracierta AP and Whiting DA.
Journal of the Chemical Society. Perkin Transactions 1, 10, 1257-1263 (1978)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service