332992
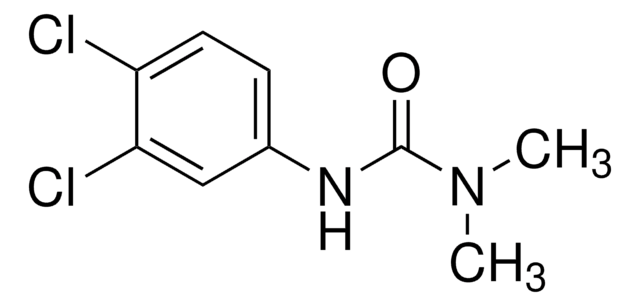
3-(4-Chlorophenyl)-1,1-dimethylurea
99%
Synonym(s):
Monuron
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
ClC6H4NHCON(CH3)2
CAS Number:
Molecular Weight:
198.65
Beilstein:
2097922
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
99%
mp
173-174 °C (lit.)
SMILES string
CN(C)C(=O)Nc1ccc(Cl)cc1
InChI
1S/C9H11ClN2O/c1-12(2)9(13)11-8-5-3-7(10)4-6-8/h3-6H,1-2H3,(H,11,13)
InChI key
BMLIZLVNXIYGCK-UHFFFAOYSA-N
Gene Information
human ... EPHX2(2053)
mouse ... Ephx2(13850)
Looking for similar products? Visit Product Comparison Guide
General description
3-(4-Chlorophenyl)-1,1-dimethylurea is an inhibitor of photophosphorylation. The kinetics of photo-induced transformation of 3-(4-chlorophenyl)-1,1-dimethylurea has been investigated in aqueous solution containing nitrates and nitrites at 310nm and 365nm. Degradation of Monuron (3-(4-chlorophenyl)-1,1-dimethylurea) photoinduced by Fe(III) in aqueous solution has been reported.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
STOMATAL RESPONSES TO CHANGES IN CARBON DIOXIDE CONCENTRATION IN LEAVES TREATED WITH 3-(4-CHLOROPHENYL)-1, 1-DIMETHYLUREA.
Allaway WG and Mansfield TA.
The New phytologist, 66(1), 57-63 (1967)
W Chu et al.
Chemosphere, 86(11), 1079-1086 (2011-12-30)
A comprehensive study of the degradation of monuron, one of the phenylurea herbicides, was conducted by UV-Vis/WO(3) process. It was found that hydroxyl radicals played a major role in the decay of monuron while other radicals (e.g. superoxide) and hole
Malathi Srinivasan et al.
Journal of biosciences, 31(5), 599-605 (2007-02-16)
Various urea-derived herbicides and different cytokinin analogues were used to determine their effects on callusing response and shoot regenerating capacity of alfalfa (Medicago sativa L.) and Coleus (Coleus forskohlii Briq.). The herbicides monuron and diuron evoked profuse callusing response from
Influence of five phenylurea herbicides on the diatom Hantzschia in a sandy loam soil.
A E Pipe et al.
Bulletin of environmental contamination and toxicology, 33(4), 439-443 (1984-10-01)
Hui Li et al.
Environmental science & technology, 38(20), 5393-5399 (2004-11-17)
Pesticide adsorption by soil clays can be dramatically influenced by the exchangeable cations present. Among the common exchangeable base cations in soils (Ca2+, Mg2+, K+, and Na+), K+-saturated clays frequently demonstrate the strongest affinity for pesticides. In the presence of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service