167177
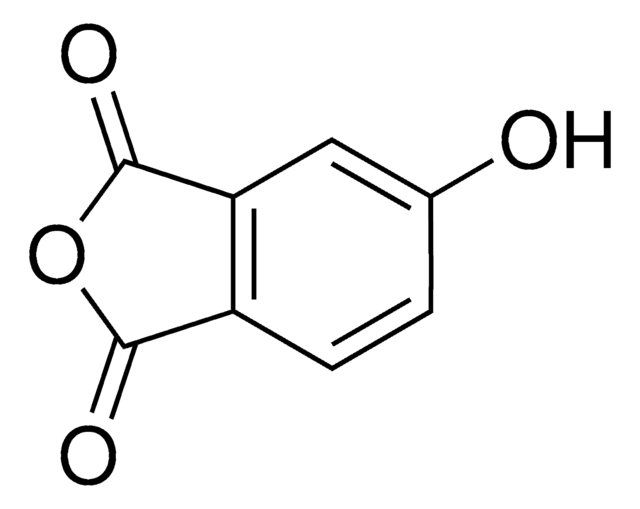
5-Hydroxy-2(3H)-benzofuranone
97%
Synonym(s):
2,5-Dihydroxyphenylacetic acid lactone, Homogentisic lactone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H6O3
CAS Number:
Molecular Weight:
150.13
Beilstein:
128621
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
powder
mp
192-194 °C (lit.)
SMILES string
Oc1ccc2OC(=O)Cc2c1
InChI
1S/C8H6O3/c9-6-1-2-7-5(3-6)4-8(10)11-7/h1-3,9H,4H2
InChI key
POUITAHNNRJWMA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
5-Hydroxy-2(3H)-benzofuranone participates in lewis acid mediated synthesis of 2(3H)-benzofuranones.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Lewis acid promoted synthesis of 2 (3H)-benzofuranones.
Babu BR, et al.
Indian J. Chem. B, 45(11), 2546-2546 (2006)
Hagai Tavori et al.
Bioorganic & medicinal chemistry, 16(15), 7504-7509 (2008-06-24)
Paraoxonase1 (PON1) is a HDL bound enzyme and many of the anti-atherogenic properties of HDL are attributed to PON1. The enzyme precise mechanism of protective action and its endogenous substrate remain elusive. PON1 hydrolyzes organophosphates, arylesters and lactones, whereas the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service