Rapid and Efficient Esterification of Fixed Oils and Fats for Fatty Acid Methyl Ester analysis by Gas Chromatography
Raj Mahat, Lance Kailey
Merck
Section overview:
Introduction
Vegetable fixed oils and fats have multiple valuable applications in food, cosmetics, pharmaceutical preparations, biofuel (biodiesel) production, and other areas.1,2 In pharmaceutical industry, vegetable oils are used as an excipient to achieve improved stability and bio-availability and thus better consumer acceptability. They are used as a green solvent in drug formulations and extraction of bio-active compounds. Type I hydrogenated oils, which have high melting point and low iodine values, are used as lubricant in tablet and capsule formulation and as an alternative to hard waxes in topical pharmaceutical formulations.3 Furthermore, vegetable oil generated nanostructured lipid particles can also be used as a carrier in lipid-based formulations. Besides having nutraceutical values, vegetable oils also have a number of pharmacological and therapeutic applications, such as antimicrobial, antitumor, anti-inflammatory, and antioxidant agents.4
Vegetable oils and fats are liquids or semisolids which primarily consist of triacylglycerols (TAGs, ~95%), diacylglycerols (DAGs, < 5%), tocopherols and other vitamins (<1%), and phytosterols (~1%).5 The main component, TAGs, are made up of three fatty acyl chains substituted to a glycerol backbone. The fatty acyl chains can have 6-24 carbon atoms and be either saturated or unsaturated. Saturated fatty acids do not have a double bond while monounsaturated fatty acids have one double bond and polyunsaturated fatty acids have two or more (up to 6) double bonds.6 A TAG can have three of the same or different fatty acyl chains. In Figure 1 is a typical TAG consisting of stearyl (C18:0), stearyl (C18:0), and oleyl (C18:1n9cis) fatty acyl chains shown.

Figure 1.Structure of a typical TAG with stearyl, stearyl, and oleyl (SSO) fatty acyl chains.
Fatty acid profiling is one of the most common ways of vegetable oil analysis. In pharmaceutical industries, USP general chapter <401> for Fats and Fixed Oils is a common way to conduct the esterification process.7 However, this method suffers from a number of limitations. First, it is a two-step esterification procedure that uses toxic chemicals, such as boron trifluoride (BF3). Second, and perhaps the most important one, from a practical standpoint, the method uses refluxing such that parallel sample preparation requires investment in the glassware’s, such as condenser, round bottom flasks, etc., and lab space.7 As a result, the method might be low-throughput and it may not be applicable for routine QC analysis.
The objective of this application note thus is to develop and validate a simple, safe, and complete alternative esterification method that is highly accurate, consistent, and capable of parallel processing for esterification of multiple vegetable oil and fat samples and also has applicability in quality control (QC) process.
Experimental
Method
Development of an alternative esterification method
- A single-step, acid-catalyzed esterification process that utilizes anhydrous acetyl chloride solution (2%, v/v) in methanol to make methanolic hydrochloride reagent was improved and validated for vegetable oils.8
Methanolic HCl Preparation
- To prepare the methanolic hydrochloride reagent solution, 2 mL of acetyl chloride (00990) was mixed with 18 mL of methanol (34860). The resulting mixture produces approximately 2 M methanolic hydrochloride (anhydrous).
Caution should be taken when adding acetyl chloride to methanol. Sudden contact with water and alcohols should be avoided. This is a highly exothermic process and can build heat and pressure if not handled appropriately. To avoid the hazard, about 5 mL of methanol was added to a 40 mL amber vial with a PTFE septum lined cap. Acetyl chloride was added slowly with constant mixing and cooling of the vial in cold water. The pressure was vented slowly, and the vial was cooled throughout the addition of acetyl chloride. Once all the acetyl chloride was added, the rest of the methanol (~13 mL) was added into the mixture. The vial was sealed and stored free from exposure to water. The reagent can be saved for future use also if stored in a refrigerator in a properly sealed air-tight container.
Esterification reaction
- About 25 mg of neat corn oil sample (PHR2897) and 2 mL of the methanolic hydrochloride reagent were mixed in a clear glass reaction vial with conical bottom. The reaction mixture was then heated in a digital two-block heater at 80 °C for 20 min. The reaction vials were then allowed to cool to room temperature. An acid-catalyzed general esterification reaction of a TAG to produce fatty acid methyl esters is shown in Figure 2.
![Acid-catalyzed methyl esterification of TAG Chemical reaction showing acid-catalyzed methyl esterification of a tristearin triacylglycerol having stearyl (C18:0), stearyl (C18:0), and stearyl (C18:0) [SSS] fatty acyl chains with three moles of methanol in the presence pf H+ ions, 80 ˚C, and for 20 minutes to produce three moles of methyl stearate and glycerol.](/deepweb/assets/sigmaaldrich/marketing/global/images/technical-documents/articles/pharma-and-biopharma-manufacturing/small-molecules-analysis-quality-control/figure-2-acid-catalyzed-methyl-esterification/figure-2-acid-catalyzed-methyl-esterification.jpg)
Figure 2.Acid-catalyzed methyl esterification of a TAG with stearyl (C18:0), stearyl (C18:0), and stearyl (C18:0) [SSS] fatty acyl chains to produce methyl stearate.
Extraction
- To carry out the liquid-liquid extraction, 2 mL of 6% (w/v) Na2CO3 (222321) solution in water was added to the cooled reaction mixture and vigorously shaken. One volume of 2 mL n-heptane (650536) was added as the extraction solvent. After adding heptane and shaking, the mixture was allowed to stand at room temperature until the phases had completely separated. The hydrophobic fatty acid methyl esters partitioned into upper heptane phase, of which 1 mL was carefully transferred to an autosampler vial and 1 µL was injected on to a GC-FID (Agilent, 7890 A GC) equipped with a Supelco® Omegawax column 30 m x 0.53 mm ID, 0.5 µm (25374) for chromatographic separation. The general workflow for the esterification and detection process is shown in Figure 3. The details of the GC-FID detection method are presented in Table 1 and a representative chromatogram of a corn oil FAME profile is shown in Figure 4.




Figure 3. Representation of esterification process workflow followed by a GC-FID detection. 3a. A glass vial containing approximately 25 mg of oil and 2 mL of reagent. 3b. Glass vials placed in a digital two-block heater set at 80°C for 20 minutes. 3c. A separating funnel containing the cooled reaction mixture, along with 2 mL of 6% (w/v) Na2CO3(aq) and 2 mL of heptane. 3d. Injection of 1 µL of content from the top layer into GC-FID instrument.

Figure 4.A representative GC-FID chromatogram showing FAME components in corn oil.
Results and Discussion
Method Validation: Quantitative esterification process
A corn oil secondary pharma standard (Cat. No. PHR2897) in five different amounts in the range of 10-35 mg was esterified and analyzed by GC-FID. The corresponding FID signals (peak area) were plotted against the amount of oil. A separately esterified oil sample having weight about 25 mg was injected 10 times to calculate precision. About 25 mg of a pharmacopeia primary standard for corn oil was esterified using the same alternative method and used as a reference standard for estimation of accuracy. Acceptance criteria for method validation parameters were referenced from USP <401> and general practices adopted while following compendial methods. Accuracy was chosen to be within 98-102%. Precision or repeatability, in term of RSD%, was set below 5% for the individual FAMEs and below 1% for the ratio of methyl palmitate and methyl stearate (C16:0/C18:0).7 Linearity criterion was set as regression coefficient, R2, above 0.99. Chromatographic resolution of at least 1.5 between methyl stearate (C18:0) and methyl oleate (C18:1) peaks was the criterion for specificity.
In brief, accuracies for two predominant FAMEs, methyl oleate (C18:1) and methyl linoleate (C18:2), were 101.3% and 99.8% respectively (Table 2). RSDs for all the individual FAMEs were below 5% and the RSD for methyl palmitate to methyl stearate ratio (C16:0/C18:0) was 0.2% (Table 3). Linear detector responses were observed for oil sample in the amount of 10-35 mg with r2 > 0.99 for all individual FAMEs present. For instance, R2 of 0.996 was found for methyl palmitate (C16:0) (Figure 5). Limit of detection (LOD) and limit of quantitation (LOQ) were estimated from LINEST function to be 2.36 mg and 7.87 mg in term of the amount of the oil sample, which correspond to 1.18 mg/mL and 3.94 mg/mL respectively in term of concentration (Table 4). The resolution between the two peaks, C18:0 and C18:1, was 3.4.
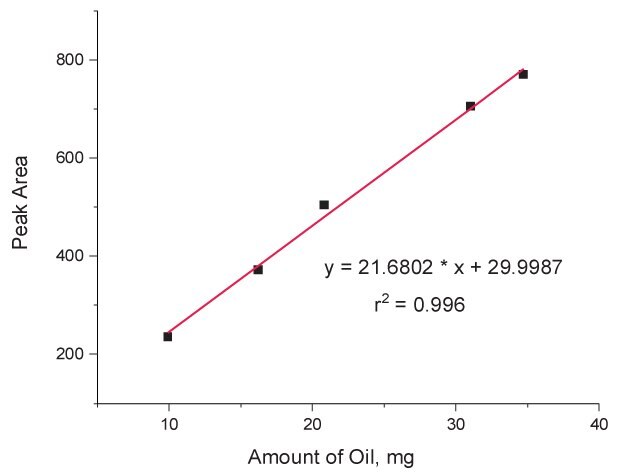
Figure 5.Calibration curve showing linear detector (GC-FID) response for the sample amount in the range of 10-35 mg of corn oil using methyl palmitate (C16:0) as the representative FAME.
Efficiency of esterification reaction: evaluation of completeness of the reaction
The completeness of the esterification process, and efficiency of the reaction, was evaluated qualitatively. An incomplete/inefficient esterification process partially hydrolyzes/esterifies TAGs producing free fatty acids and MAGs and DAGs. So, presence of such lipid classes was taken as markers for the incomplete esterification process.
For this, about 35 mg of corn oil samples were esterified with 1 and 2 mL of the methanolic hydrochloride reagent in parallel. The extractions were carried out with 2 mL of heptane at acidic pH with prior addition of 2 mL of water instead of Na2CO3 solution. About 500 µL of each extract was subjected to another derivatization step with an addition of 100 µL of MSTFA reagent (69479) and heating for 15 min at 70 °C. This derivatization step replaces the acidic protons from the carboxylate and hydroxyl functional groups with a trimethylsilyl (TMS) moiety converting unesterified free fatty acids into –TMS ester and MAGs and DAGs into –(n)TMS ether derivatives. Here, n equals to number of hydroxyl functional group(s) in the molecular structure. A general scheme of a silylation reaction for a partially hydrolyzed monoacylglyceride, such as 1-palmitin, and a free fatty acid, such as palmitic acid, is shown in Figure 6.
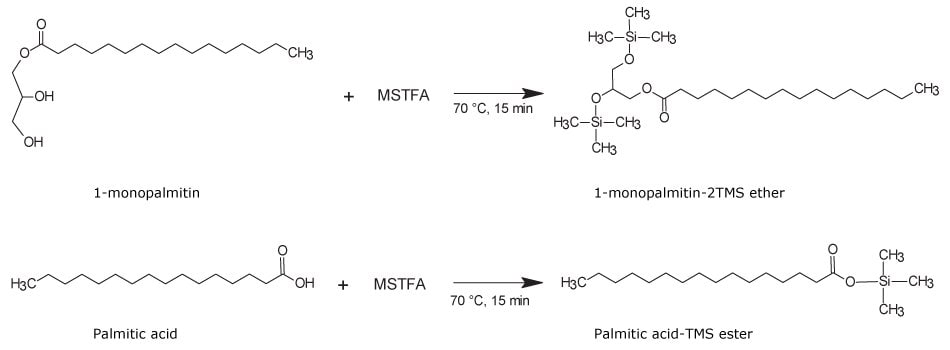
Figure 6.Trimethylsilylation derivatization of a partially esterified monoacylglycerol, such as 1-monopalmitin, to produce 1-monopalmitin-2TMS ether derivative (top reaction). A non-esterified free fatty acid, such as palmitic acid, produces palmitic acid-TMS ester (bottom reaction).
The reaction mixture was analyzed on a single quadrupole GC-MS (Agilent, 5975C MSD) in full-scan mode in the m/z range of 40-550 with a solvent delay of 3.5 min. The chromatographic separation was carried out on a SLB®-5ms (30 m x 0.25 mm x 0.25 µm, 28471-U) capillary column. An aliquot of 1.0 µL of the sample was injected without removal of the excess MSTFA reagent in the injector heated at 220 °C with split ratio of 10:1. Injection of silylation reagent is safe on GC columns that do not have free hydroxyl group. Said that, columns containing PEG stationary phase, such as Omegawax, Carbowax, Nukol, etc., should be avoided. The GC oven was set at 70 °C for 2 min and then ramped at the rate of 5 °C/min to final temperature of 240 °C which was held for 5 min. Helium was used as carier gas at the constant flow rate of 1 mL/min. The ionization energy of 70 eV was used to collect EI spectra at the sampling rate of 4 scans/sec. The transfer line, ionization source, and the quadrupole anlayzer were set at 240 °C, 230 °C, and 150 °C, respectively.
As shown in Figure 7, careful inspection of the chromatogram (top) for the oil sample esterified with 1 mL of the methanolic hydrochloride showed presence of 1-monopalmitin-2TMS, 1-monoolein-2TMS, and 1-monolinolein-2TMS derivatives. This signified incomplete esterification process with 1 mL of the esterification reagent. On the other hand, absence of such peaks on the bottom chromatogram suggested completeness of the process with 2 mL of the reagent. This concluded, 2 mL of the methanolic hydrochloride reagent is sufficient for complete esterification of the oil samples up to 35 mg.
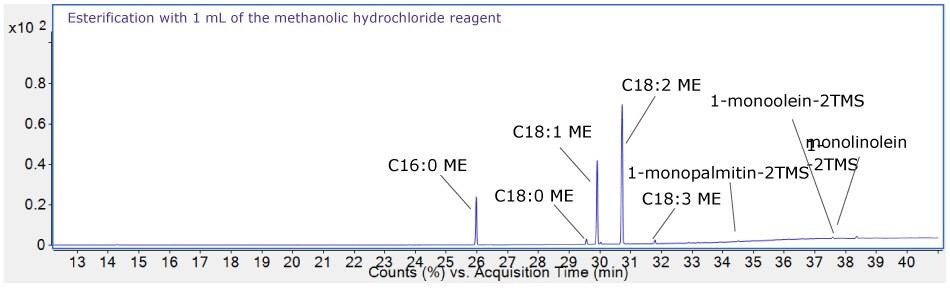
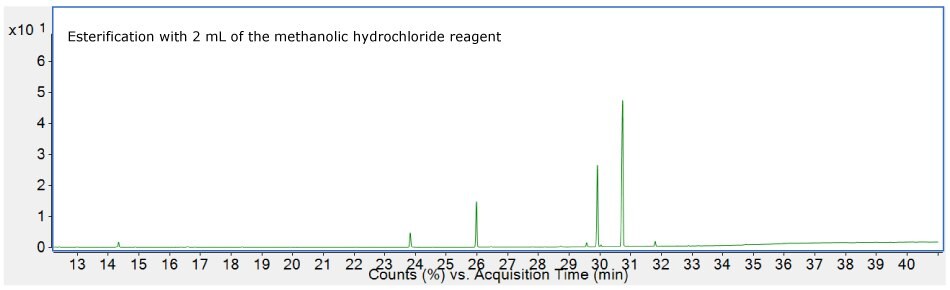
Figure 7.Chromatograms showing efficiency of the esterification process with 1 mL (top) and 2 mL (bottom) reagent.
Replication of the esterification process for analysis of olive oil samples
The esterification process was replicated for analysis of other oil samples. Figures 8 & 9 shows the complete qualitative agreement between olive oil pharmaceutical secondary standard (PHR2902) and the pharmacopeia reference standard.
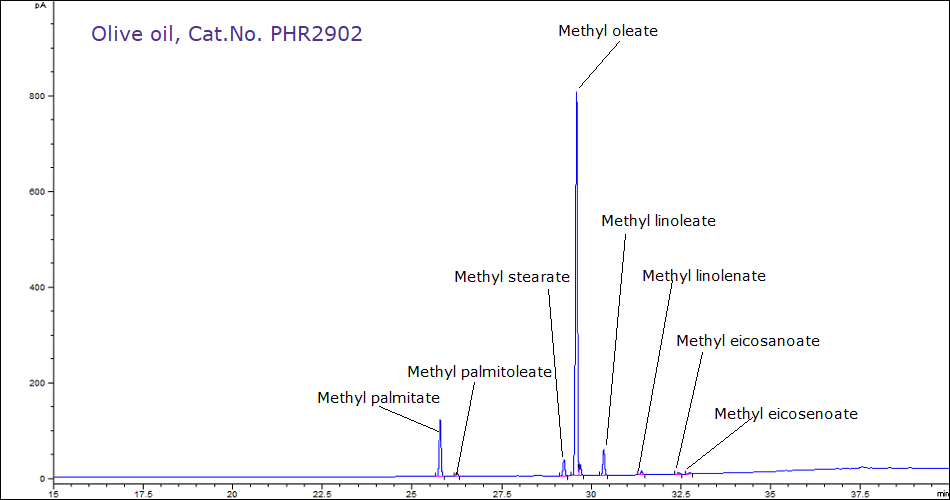
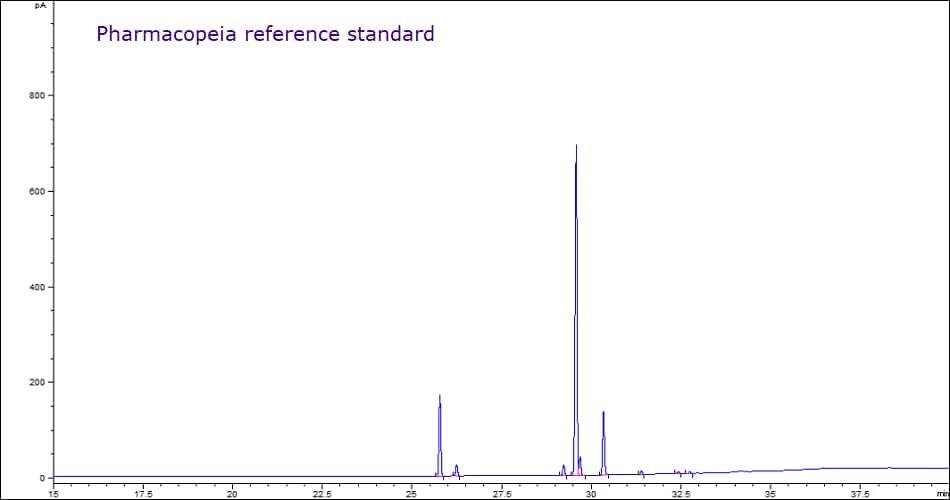
Figure 8.Comparative gas chromatograms showing equivalent FAME profiles of olive oil samples sourced from MilliporeSigma (PHR2902) and a Pharmacopeia reference standard.
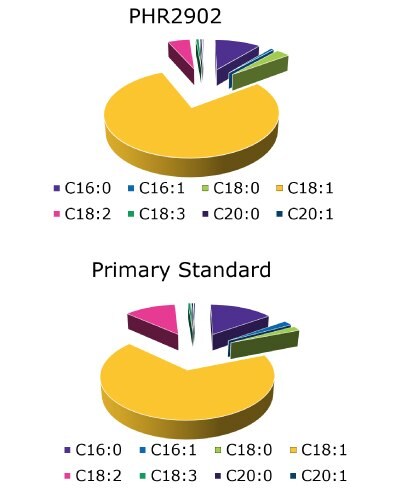
Figure 9.Pie-charts showing actual FAME contents of the both standards following USP guidelines.
Application of the method in esterification of other vegetable oil and fat samples
The method was used in the methyl esterification of 18 fixed oil and fat samples. Replicate sample processing was carried out for each oil sample. The applicability of the method for QC of the multiple oil samples was examined by conducting the esterification of the corresponding primary standards. High qualitative and quantitative FAME profile agreement between the PHR and pharmacopeia reference standards shows method‘s QC applicability. Summary of FAME profiles of the vegetable fixed oil and fat samples is presented in Table 5.
Conclusion
The developed and validated fatty acid methyl esterification method that utilizes methanolic hydrochloride reagent prepared from 2% (v/v) acetyl chloride solution in methanol is quantitative, simple, complete, and high-throughput allowing parallel sample processing. As many as 24 samples can be processed in parallel, within 20 min. The method is able to esterify various oil and fat samples including complex oil samples, such as fish oil. The method has high QC applicability. Strong qualitative and quantitative agreements in the results of analyses of multiple vegetable fixed oils and fats in parallel with the respective pharmacopeia reference standard confirm method‘s applicability in QC laboratories. This method can be used as an alternative to USP <401> in characterization/FAME analysis of vegetable oils and fats in pharmaceutical and food industry laboratories.
REFERENCES
Um weiterzulesen, melden Sie sich bitte an oder erstellen ein Konto.
Sie haben kein Konto?