Verkade's Bases
Strong and Hindered Bases in Organic Synthesis
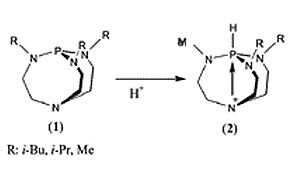
Verkade's bases, football-shaped proazaphosphatrane molecules of type 1, are strong bases due to the extraordinary stability of 2 when 1 reacts with a proton.
Due to the stability of the protonated form 2, Verkade's bases are about eight orders of magnitude stronger as a Lewis base than any amine known, including the prominently used DBU, DBN and Proton Sponge (Product No. 14795).
Applications: Verkade's bases have been successfully applied in a variety of organic reactions, such as alkylations, dehydrohalogenations, acylations,1 a variety of condensation and organometallic reactions for carbon-carbon bond formation.2 A second characteristic of the novel cage molecules of type 1 is their ability to act as a superior catalyst for a continuously widening range of reactions3-6 such as protecting alcohol groups with various silyl groups during multistep syntheses,4 trimerizing isocyanates to isocyanurates5 and the synthesis of alpha, beta-unsaturated nitriles.6
References
Um weiterzulesen, melden Sie sich bitte an oder erstellen ein Konto.
Sie haben kein Konto?